As a promising alternative energy storage technology, rechargeable aqueous zinc metal batteries (RAZMBs) have received extensive attention due to their abundant natural resources, inherent safety and cost-effectiveness.Nevertheless, Zn metal anodes suffer from detrimental drawbacks including non-uniform electrochemical plating and inevitable side-reactions of corrosion, resulting in its poor electrochemical performance, which constitute the bottlenecks for scale-up utilization of RAZMBs. In pursuit of high-performance Zn metal anodes for RAZMBs, various strategies including surface protection, nanostructure design, and electrolyte optimization have been proposed to stabilize metallic Zn during electrochemical cycling. In particular, concentrated or "water-in-salt" electrolytes became one of the most important strategies by increasing the availability of Zn2+ions, decreasing the content of free water. Despite these exciting advancements, the issues of high cost, high viscosity, and low ionic conductivity for concentrated electrolytes have not yet been solved.
Recently, Professor Yongming Sun's research group has developedan alloying-dealloying method to prepare nanoporous Zn electrode with controlled pore size.This work enabled interface-localized concentrated electrolyte at a 3D nanoporous Zn (npZn) metal electrode by designated use of the space charge effect in the accurately controlled nanopore structure, as confirmed by both theoretical simulation and spectroscopic studies. The npZn electrode with interface-localized concentrated electrolyte showed uniform Zn plating behavior and suppressed side reactions simultaneously. As a consequence, the npZn electrode exhibited high electrochemical reversibility for 750 h under the measurement with a combination of electrochemically Zn stripping/plating cycling (1 mA cm-2, 1 mAh cm-2, 25 cycles) and resting (50 h), and looping. In contrast, the pristine Zn electrode failed after30 h of cycling under the same test condition. Moreover, a npZn||NaVO3cell showed a high capacity of 200 mAh g-1and a long lifespan with considerable capacity retention (76 % for 1500 cycles), which outperformed the pristine Zn||NaVO3cell (failed after 600 cycles due to short-circuit). These results suggested a promising route for the stabilization of Zn metal anode inrechargeable aqueous batteries.
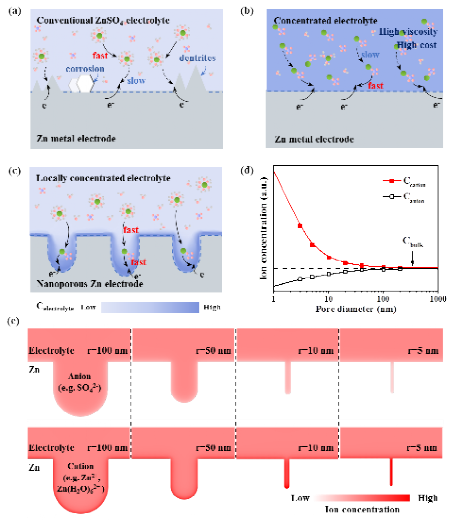
Figure 1.Schematic illustrationof Zn plating processes on (a) Zn metal electrode with conventional ZnSO4electrolyte, (b) Zn metal electrode with concentrated electrolyte, and (c) nanoporous Zn electrode with interface-localized concentrated electrolyte. (d) The ion concentration at the electric double layer of nanoporous Zn metal with different pore diameters based on Poisson-Nernst-Planck equations. (e) Surface charge densities of the cations and anions at the interface of ZnSO4 electrolyte and nanoporous Zn metal with different pore diameters ranging from 5 nm to 100 nm.

Figure 2.(a) Schematic illustration of the preparation of npZn electrode.(b) Top-view SEM image of the npZn electrode. The inset showed the digital picture of the as-prepared npZn electrode. (c)Size distribution of the macropores and mesopores of the npZn electrode. (d) Cross-section SEM and EDS elemental mapping images and (e) XRD pattern of the npZn electrode after immersing in ZnSO4 eletrolyte and washed with deionized water for 5min.(f) Raman analysis of the ZnSO4 electrolyte configuration in and outside the 3D nanoporous channels of the npZn electrode.

Figure 3.(a)Voltage profiles of the pristine Zn and npZn electrode under dynamic measurement with a combination of electrochemical cycling (1 mA cm-2and 1 mAh cm-2, 25 cycles) and resting (50 h), and looping.Galvanostatic charge/discharge curves of (b) pristine Zn||NaVO3 and (c) npZn||NaVO3 cells at the 1st cycle and the 602nd cycle.(d) Cycling performance of pristine Zn||NaVO3 and npZn||NaVO3 cells at a current density of 1 mA cm-2.(e) Top-view SEM image of the pristine Zn electrode after 100 cycles at 1 mA cm-2and 1 mAh cm-2.(f) Top-view and (g) cross-section SEM images of the npZn electrode after 100 cycles at 1 mA cm-2and 1 mAh cm-2.
Related work has been published on Chemical Engineering Journal(https://authors.elsevier.com/sd/article/S1385-8947(21)01228-6)on April 5, 2021 with the title of "Localizing concentrated electrolyte in pore geometry for highly reversible aqueous Zn metal batteries". The first unit for this research is Wuhan National Laboratory for Optoelectronics Huazhong University of Science and Technology.