Due to their considerable energy density, abundance of Na resources, and potentially low cost,Na metal batteriesare promising battery systems for various energy applications in modern society, such as electric vehicles and grid energy storage. Recently, Na metal batteries with solid Na metal anode and liquid organic electrolyte have been widely investigated at room temperature. However, poor processability of metallic Na requires complicated electrode fabrication technology, and high chemical reactivity lead to safety concerns, low Coulombic efficiency (CE) and inferior cycling stability.When the battery is heated or the ambient temperature rises, the side reaction between sodium metal and electrolyte becomes more serious, which seriously hinders the development of sodium metal secondary battery.The side reaction between sodium metal and electrolyte becomes more seriousat elevated temperature (e.g.,≥60 ºC), which seriously hinders the development of Na metal batteries.
Recently, Professor Yongming Sun's group at Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, developed a Na15Sn4/Na composite featuring uniform mixture of metallic Na and Na15Sn4network through a simple coldcalendaringapproach using metallic Na and Sn as the initial materials, where Na15Sn4isin-situgenerated and implanted in Na metal matrix due to the spontaneous alloy reaction (4Sn+15Na → Na15Sn4) during the repeated calendaring process(Figure 1a).The Na15Sn4/Nacomposite shows much better processability than the pure metallic Na due to the enhanced mechanical property from alloying (Figure 1b,c,dand e).It not only endows the electrode with uniform electron and Na+fluxes on the electrode to avoid the growth of dendrites, but also acts as a stable host to alleviate the problem of volume change, and thus maintain the integrity of the electrode and suppress the generation of thick SEI during the stripping/plating cycling of metallic Na.As expected, the as-fabricated Na15Sn4/Na electrode exhibited stable cycling with overpotential of 15 mV for 100 cycles in symmetric cells at 60ºCunder 1 mA cm-2and 1 mAh cm-2. In contrast, short circuit took place after only 8 cycles for bare metallic Na under the same test condition. Moreover, good electrochemical stability was also achieved for the symmetric cell cycled at 90ºC(Figure 1f).
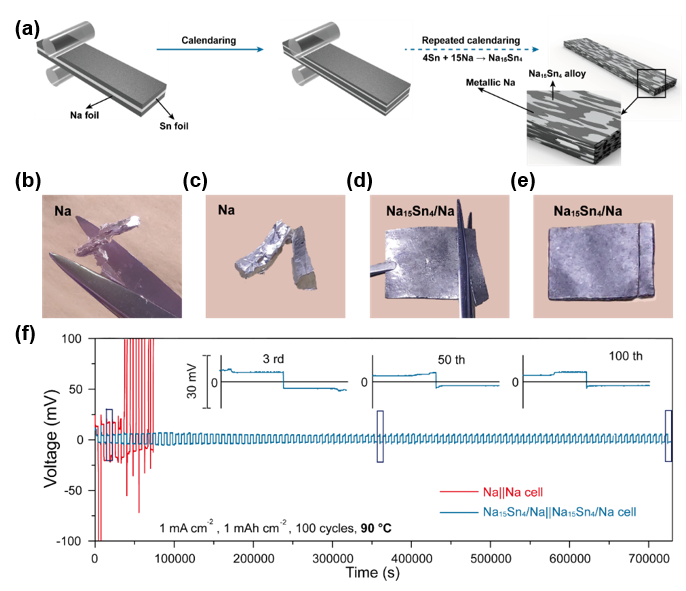
Figure 1.(a) Schematic of the formation ofNa15Sn4/Nacompositefoil.(b,c)Digital camera images ofNaduring tailing (b) and aftertailingapart (c).(d,e) Digital camera images of as-fabricated Na15Sn4/Na foil during tailing(d)and after tailing into designed geometric shapes (e). (f)Galvanostatic cycling of Na15Sn4/Na||Na15Sn4/Na and Na||Na cells under current density of 1 mA cm-2with the capacity fixed at 1 mAh cm-2at 90ºC.
This work is reported on Energy Storage Materials named " Enhanced processability and electrochemical cyclability of metallic sodium at elevated temperature using sodium alloy composite" (Energy Storage Materials, 2021, 35, 310-316. https://doi.org/10.1016/j.ensm.2020.11.015).Guocheng Liat Wuhan National Laboratory for Optoelectronicsisthe first author.Prof.Yongming Sun lead this work. Prof.Bao Zhang, Jianjun JiangandHui YangatHuazhong University of Science and Technology, Prof.Li WangatTsinghua University, Prof.Zhi Wei Sehat A*STARalso contributed to this work.