Rechargeable lithium-ion batteries (LIBs) are boosting a flourishing era of portable electronic devices and zero-emission electric vehicles and they also exhibit great potential for the storage of renewable energy, such as solar, geothermal, water, tidal and wind power. In the existing LIBs, intercalated lithium oxide or phosphate cathodes provide lithium ions for battery cycling. Although great improvement has been achieved in the electrochemical performance of intercalated cathode materials, further advancement in energy density of batteries is hindered by their limited specific capacities (typically < 200 mAh g-1). Alternatively, lithium-free cathode materials typically deliver much higher theoretical capacities than intercalated lithium oxide materials, such as 1672 mAh g-1 for sulfur, 441 mAh g-1 for V2O5 and 712 mAh g-1 for FeF3. Thus, much higher energy density can be expected for LIBs using these lithium-free cathode materials, once they are successfully paired with high-capacity lithium-containing anode materials.
In view of such an intension, Prof. Yongming Sun and co-workers reported a novel high-capacity lithium-containing Li3P/C nanostructure anode material, consisting of an electrochemically active Li3P nanodomains embedded in a porous carbon matrix with particle size of several micrometers, delivers high capacity, good rate capability and stable cycle life. As illustrated in Fig. 1, such Li3P/C nanostructure has multiple advantages: (i) an interconnected porous carbon network enables rapid electron transport, (ii) small size of Li3P nanoparticles with a short solid-phase ion diffusion length leads to fast solid-phase reaction and high utilization of active materials, and (iii) Li3P/C embedded structure confines the volume change during charge/discharge within the nanopores of the carbon particles and thus avoids the collapse of the overall composite particle and electrode structure, leading to the formation of a stable SEI layer during cycling.

Figure 1. Schematic of Li3P/C nanocomposite for high-capacity lithium-containing battery anode. Li3P nanoclusters are embedded in the nanopores of the carbon particles. The interconnected carbon framework of the porous carbon works as the conductive skeleton for fast electron transport. The ultrafine particle size of Li3P enables fast electrochemical reactions. The volume change of Li3P/P active material is confined within the nanopores of the carbon particles and stable SEI layer is formed at the outer surface of the Li3P/C composite particle during cycling.
Benefited from the unique micro/nanostructure of the Li3P/C nanocomposite, stable electrochemical cycling with high capacity was achieved (Figure 2). The as-achieved Li3P/C nanocomposite anode provided high available lithium-ion capacity of 2179.5 mAh g-1 (calculated based on the mass of red P) at 0.1 C during the initial delithiation process. Meanwhile, the Li3P/C nanocomposite showed 75% of its 0.5 C capacity at 6 C and stable cycling for 150 cycles with 90% capacity retention at 0.2 C (1564 mAh g-1 for the 150th cycle).
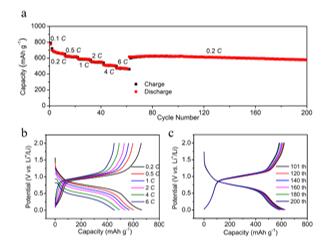
Figure 2. (a) Cycling of the Li3P/C electrode cycled at various lithiation current densities with a constant delithiation current density of 0.2 C and ((b) and (c)) the corresponding voltage-capacity plots. The galvanostatic charge/discharge measurement for Li3P/C||Li metal cells was carried out with the cut-off potential range of 0.01–2 V.
In the future, the high-capacity Li3P/C composite can be paired with high-capacity lithium-free battery cathodes (e.g., sulfur, V2O5, and FeF3) to produce next-generation lithium-ion batteries with high energy density. Also, the high-capacity Li3P/C composite can also work as a prelithition additive to address the initial lithium loss in lithium-ion batteries.