On October 19th, the journal“Macromolecules”published the research by Prof. Ming-Qiang Zhu from Wuhan National Laboratory for Optoelectronics and Prof. Ben Zhong Tang from The Hong Kong University of Science and Technology. The article title is“Geminal Cross-Coupling of 1,1-Dibromoolefins Facilitating Multiple Topologicalπ‑Conjugated Tetraarylethenes”.
The cross-coupling reactions have been used in general C-C bond formation to develop newπ-conjugated molecular topological configurations, which is an essential objective for material chemists and can be used extensively in optoelectronic devices such as chemical biosensing, organic light emitting diode (OLED) and organic photovoltaics. Here, we report the general twofold germinal C-C bond formation at 1,1-dibromoolefin via Suzuki or Stille cross-coupling reactions of aromatic boronic acid/ester over heterogeneous Pd catalysts for multipleπ-conjugated molecular topological configurations. We employ a series of recipes from a library of precursors to produceπ-conjugated macrocyclics, conjugated dendrimers, linear conjugated polymers and 2-3 dimensional conjugated covalent organic frameworks (COF). This universal strategy toward specificπ-conjugated molecular topological configurations enables efficient coupling of aryl bromides with various coupling partners with high activity and selectivity under mild conditions. Theπ-conjugated macrocycles, dendrimers, and linear polymers show characteristic aggregation-induced emission properties. The conjugated microporous polymers possess unique porosity of 2−3 nm. The microporous polymer nanoparticles can be redispersed in solution.
This work has been on-line published in Macromolecules(2015, 48, 7823-7835) This work was supported by the National Basic Research Program of China (Grants 2015CB755602 and 2013CB922104) and NSFC (21474034 and 21174045).
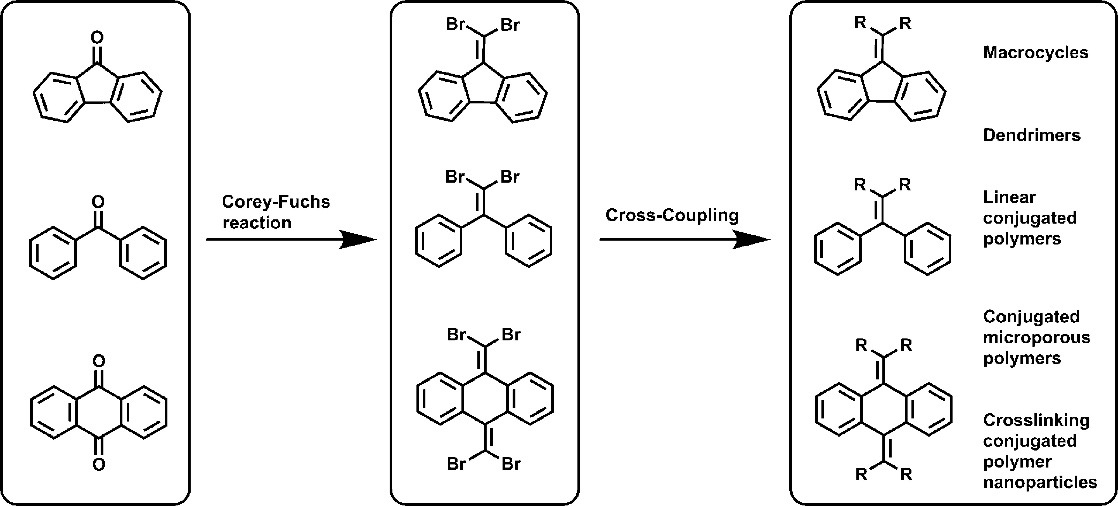
Figure 1. Typical Cross-Coupling for Multiple Topological Configuration ofπ-Conjugated Tetraarylethene Systems