Recently, Prof. Zhihong Zhang's team applied intravital optical molecular imaging technology to study the effect and mechanism of intracellular antigens (intra-Ags) induced immune clearance of liver metastasis, revealing that cell membrane pores induced by the ROS-caspase-3-GSDME pathway play an important role in the process of tumor immunotherapy targeting intra-Ags. This work was published in Theranostics (12(17):7603-7623, 2022) with the title Intravital molecular imaging reveals that ROS-caspase-3-GSDME-induced cell punching enhances humoral immunotherapy targeting intracellular tumor antigens.
The tumor antigen-induced immune response and antibodies targeting tumor antigens play an important role in the antitumor effect, and the development of tumor vaccines based on these tumor antigens is one of the important aspects of tumor immunotherapy. Tumor antigens are divided into intra-Ags and extracellular antigens. Due to the barrier of the cell membrane, intra-Ags cannot be directly recognized and bound by antibodies. One of the keys to tumor immunotherapy targeting intra-Ags is to change the permeability of cell membranes, thereby improving the recognition and binding of antibodies to intra-Ags. The molecular signaling changes in tumor cells are closely related to the permeability of the cell membrane. However, the factors that affect the permeability of the tumor cell membrane in the microenvironment and the molecular mechanism are still unclear. Therefore, observing molecular signal changes in tumor cells through intravital optical molecular imaging and studying the molecular mechanism that promotes the release of intra-Ags in tumor cells and binding to antibodies in the blood will help the development and application of tumor vaccines based on intra-Ags.
In this study, tfRFP immunization at the tail base of C57 mice had a specific humoral immune response effect on B16 tumor cells expressing the intracellular tfRFP antigen in the hepatic sinusoid and effectively inhibited the growth of liver metastases of tfRFP-B16 cells. In the early stage of B16 tumor cells injected into the hepatic sinusoids through the spleen, some tumor cells were affected by blood shear stress and metabolic changes. In vivo optical molecular imaging results showed that some circulating tumor cells in the liver displayed elevated ROS and activated caspase-3. Then, the intracellular GSDME protein is cleaved by activated Caspase-3 to form GSDME-NT, which can form holes in the cell membrane and release the intracellular antigen tfRFP. The released intracellular antigen combines with the extracellular antibody to form tfRFP antigen-antibody complexes (also known as immune complexes). The complement system is further activated by antigen-antibody complexes to form the C5b-9+ membrane attack complex, which accelerates the cell membrane punching effect and simultaneously recruits neutrophils and F4/80+ macrophages to the liver to exert a synergistic antitumor effect.
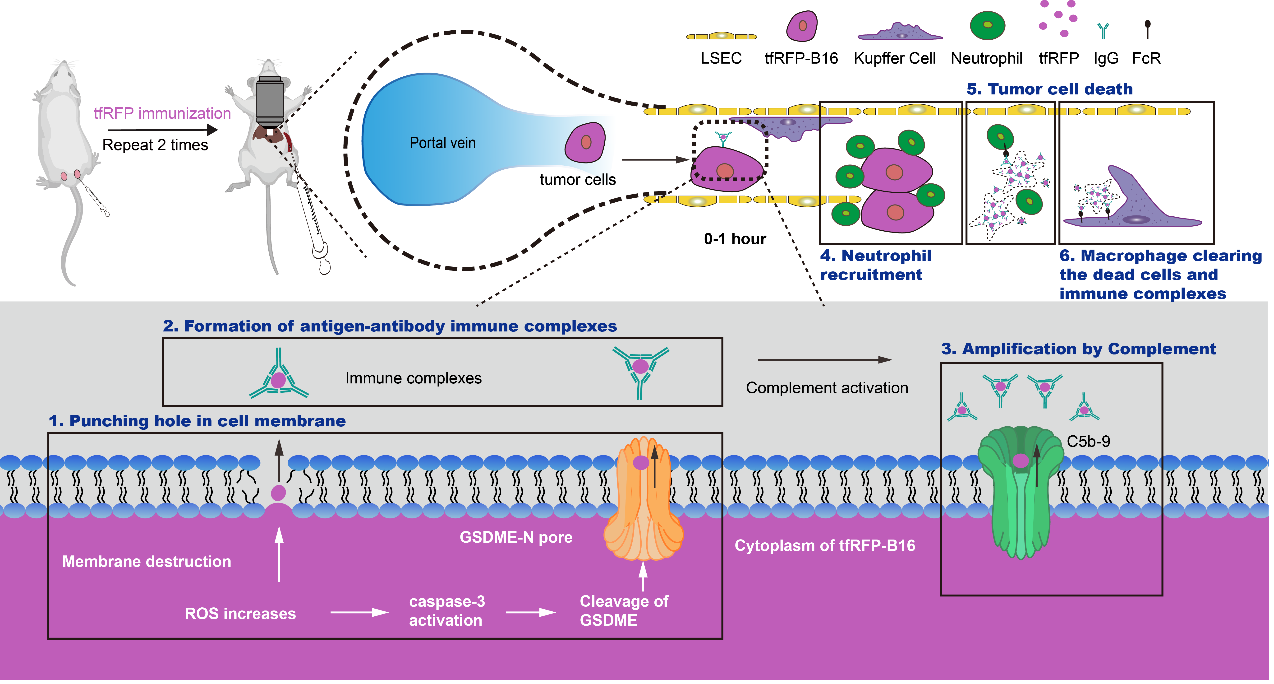
Graphical Abstract: Schematic diagram of liver metastasis elimination in the tfRFP-immunized mice was triggered through the ROS-caspase-3-GSDME pathway. Initially, elevated reactive oxygen species (ROS) in circulating tumor cells leads to slight activation of caspase-3, resulting in cleavage of GSDME proteins and perforation of the tumor cell membrane. Then, punching holes in the tumor cell membrane resulted in the release of intra-Ag tfRFP and the formation of antigen-antibody complexes. The immune complexes activate the complement component to form MACs to amplify the release of intra-Ag, and neutrophils are recruited. The immune complexes and dead tumor cells are phagocytosed by F4/80+ macrophages.
Professor Zhihong Zhang and Associate Researcher Shuhong Qi are the co-corresponding authors of the article; Bolei Dai and Ren Zhang are the co-first authors of the article; Lei Liu, Xian Zhang, Deqiang Deng, Jie Zhang, Yilun Xu, Fanxuan Liu, Zheng Liu, and Academician Qingming Luo are co-author. This work was supported by the National Key Research and Development Program of China (2017YFA0700403), the National Natural Science Foundation of China (91842305, 91842307, 81901691), the Hainan University Scientific Research Foundation (KYQD(ZR)20078), and the Innovation Fund of WNLO.