Solid-electrolyte interphase (SEI) with high stability and high Li+conductivity is highly desirable for Si-based lithium-ion batteries with high energy density and superior fast charging capability.
Recently, Professor Yongming Sun’Group proposed that constructing a superior SEI by regulating the interaction between electrolyte components and anode surfaces to achieve the above goal. The authors utilized molecular dynamics (MD) simulations and density functional theory (DFT) to investigate the adsorption behavior of fluoroethylene carbonate (FEC, a common electrolyte solvent) on alloy-type anode materials (including red-P, Si, SiO) surface. The research revealed that the FEC adsorption density on the red-P surface was higher than that on the other Si surface components, and the binding energy between the red-P and FEC was higher than those between Si and SiO surfaces. Increasing the chance of FEC molecules being absorbed into IHP is expected to improve the utilization rate of FEC, thereby assisting in forming a robust SEI on the alloy-type anode. Based on these findings, the authors successfully constructed an ultrathin nanolayer on SiOxsurfaces (SiOx@P) by means of the formation of Si-O-P chemical bonds. With an ultrathin P interphase layer, FEC was preferentially adsorbed and decomposed on the SiOxsurface, resulting in the formation of a dense symbiotic Li3P/LiF-rich SEI. In the initial cycle of battery operation, the LiNi0.6Co0.2Mn0.2O2(NCM622) ||SiOx@P pouch cell demonstrated rapid charging capability (86.5% and 81.2% of full charge within 15 and 10 minutes, respectively) and excellent cycling performance (~1.2 Ah, 250 cycles, with a capacity retention rate of 83.8% under fast charging conditions of 4C, 15 minutes). This achievement offers a unique insight into SEI formation, providing new opportunities to construct advanced SEI for Si-based anodes toward high energy density fast charging LIBs.
SEI is formed through the decomposition of the electrolyte components adsorbed on the inner Helmholtz plane (IHP) of the anode surface. Scientifically, the constitution and spatial configuration of specific adsorption behavior, which is significantly affected by the composition and properties of electrolyte components and substrate, plays a significant role in SEI formation. Despite considerable progress in electrolyte engineering, the mechanism of how the substrate interacts with the electrolyte and its effect on SEI remains unexplored. Here, we investigated the impact of different substrates on the structure and properties of SEI in carbonate electrolytes containing FEC additives, which was widely regarded as an active film-forming species and could be reduced to inert polyvinylidene carbonate (poly-VC) and high modulus LiF to enhance SEI stability.30Increasing the chance of FEC molecules being absorbed into IHP is expected to improve the utilization rate of FEC, thereby assisting in forming a robust SEI on the alloy-type anode. In this study, the adsorption behavior of FEC molecules on different surfaces of alloy-type materials (including Si, SiO, and red-P) was investigated using MD simulations and DFT calculation. The adsorption density profile of FEC molecules in the parallel orientation on the slabs of Si, SiO, and P surfaces and the binding energy on those surfaces toward FEC were considered. The results showed that the FEC adsorption density on the red-P surface was higher than that on the other Si surface components, and the binding energy between the red-P and FEC (0.18 eV) was higher than those between Si (0.09 eV) and SiO (0.17 eV) surfaces. To reveal the adsorption behavior mechanism of FEC in IHP and its impact on SEI formation, experimentally, we investigated the electrochemical behavior of the SiOx@Pand bare SiOxanodes in FEC-containing electrolyte. The linear sweep voltammetry (LSV) curve demonstrated that both the non-Faradaic double layer capacitance adsorption process and Faradaic decomposition process in the IHP took place in the range of 1.4–2.5 V and 0–1.4 V, respectively (Fig. 1e). Alternating current (AC) voltammetry was utilized to observe the adsorption behavior, and the non-Faradaic capacitance-potential curves were calculated based on the data collected (Fig.1f). It showed that both the potential and capacitance of the SiOx@P at the potential of zero charge (PZC) changed compared to the bare SiOx(2.55vs. 2.60 V for PZC), revealing their difference of FEC adsorption features in the IHP.
In general, we revealed that interphase structure could play a significant role in the formation of SEI and regulate the electrochemical performance of a high-capacity Si-based anode. By introducing a functional P interphase layer, a robust Li3P/LiF-rich SEI was successfully constructed on the SiOxsurface through its specific adsorption with the FEC additive in IHP, and it improved the mechanical and electrochemical stability during the repeated charge/discharge processes. As a result, the SiOx@P showed remarkable improvement in terms of CE, rate capability, and cycle stability compared to the bare SiOx. This work has shed light on utilizing surface engineering to tailor the composition and mechanical properties of the SEI and may boost advanced SEI design for high-energy-density LIBs with fast charging capability.
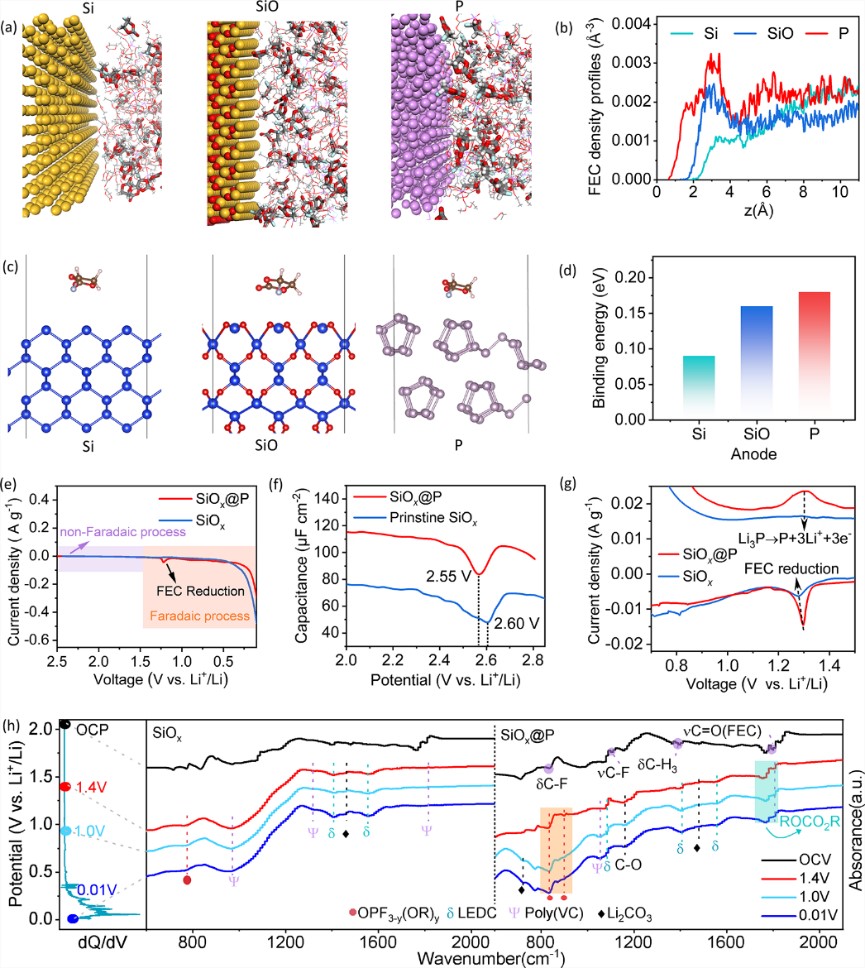
Fig.1 (a) MD simulations and (b) FEC adsorption density profiles on the Si and P surfaces. (c) DFT calculations and (d) binding energy of FEC molecules adsorption on different material surfaces. (e) The LSV curves of the SiOx@P and bare SiOx anodes. (f) The non-Faradaic capacitance-potential curves for the SiOx@P and bare SiOx electrodes at 2.0 − 3.0 V. (g) Initial CV curves of the SiOx@P and bare SiOx electrodes at a scanning rate of 0.01 mV s-1 between 0.7–1.5 V. (h) dQ/dV plot of the SiOx@P electrode and ex-situ FTIR spectra of the SiOx@P and bare SiOx electrodes at different potential during the first discharge process.
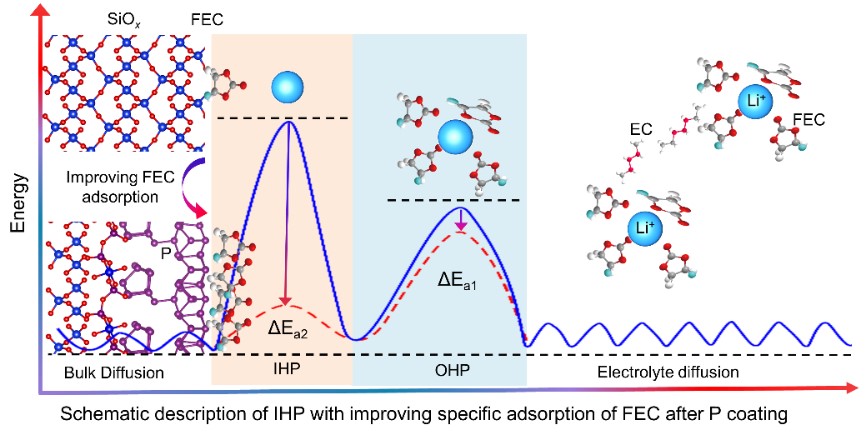
Fig. 2 Schematic description of IHP with specific adsorption of FEC.
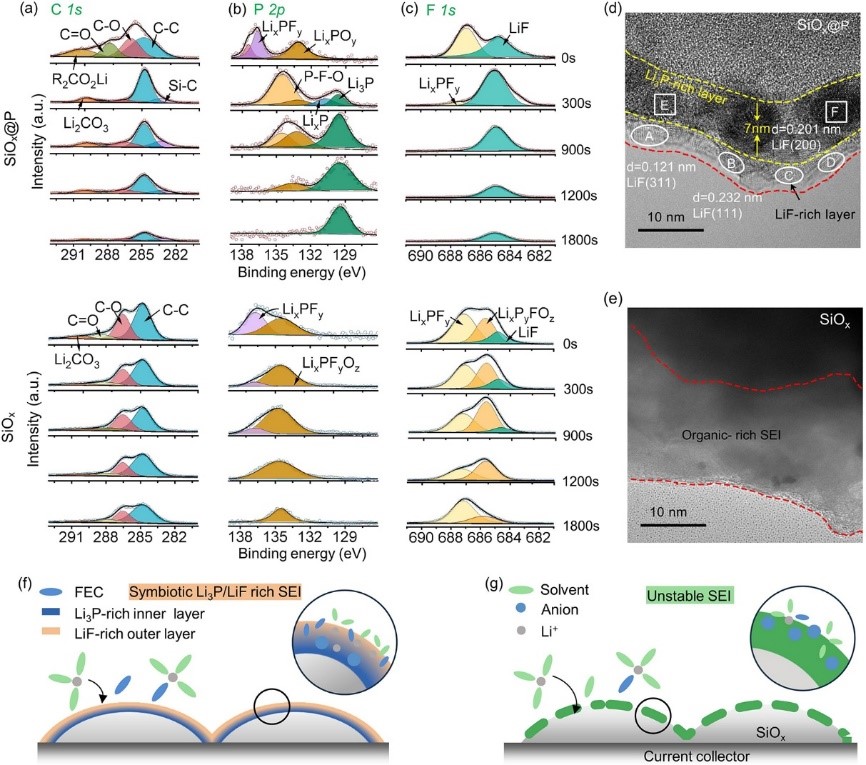
Fig. 3 (a-c) The high-resolution (a) C 1s, (b) P 2p, and (c) F 1s, XPS spectra depth profiles of the SiOx@P and bare SiOx after the formation cycle. (d and e) HRTEM images of the SiOx@P (d) and bare SiOx (e) after the formation cycle. (f and g) Schematic of the SEI formation on the SiOx@P (f) and bare SiOx (g). Selective adsorption and catalytic electrolyte decomposition on the P surface could enable the formation of symbiotic Li3P/LiF-rich SEI.
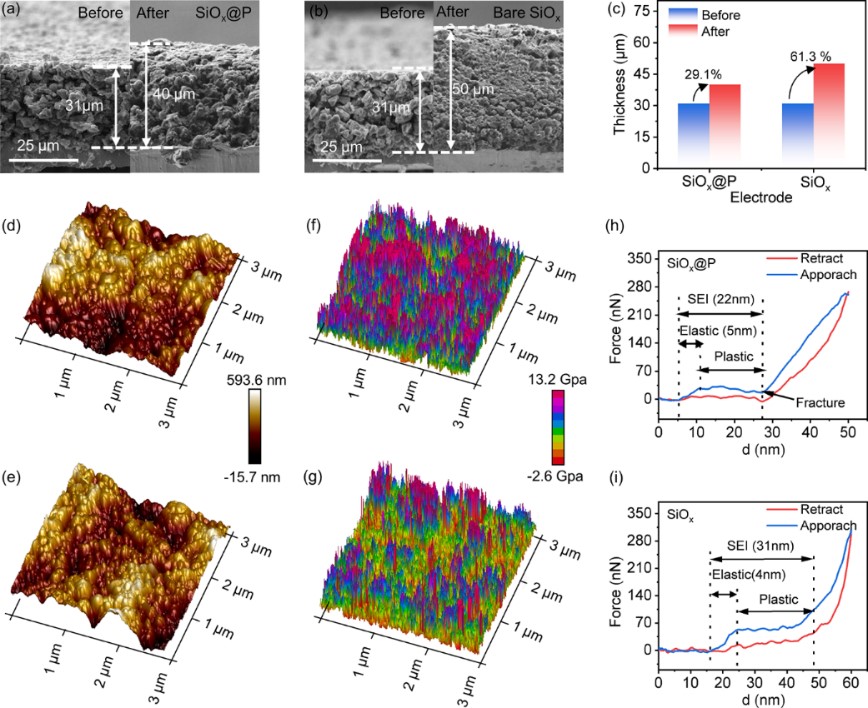
Fig. 5 (a-c) SEM images of the cross-section of the SiOx@P (a) and the bare SiOx electrodes (b) before and after 100 charge/discharge cycles at 1.0 C, and (c) comparison of the thickness variation and swelling rate of both electrodes. (d, e) Typical AFM morphology and (f, g) Young’s modulus comparison of the SEI in the SiOx@P and bare SiOx anodes after 100 charge/discharge cycles at 1.0 C. (h, i) Force-displacement curves.
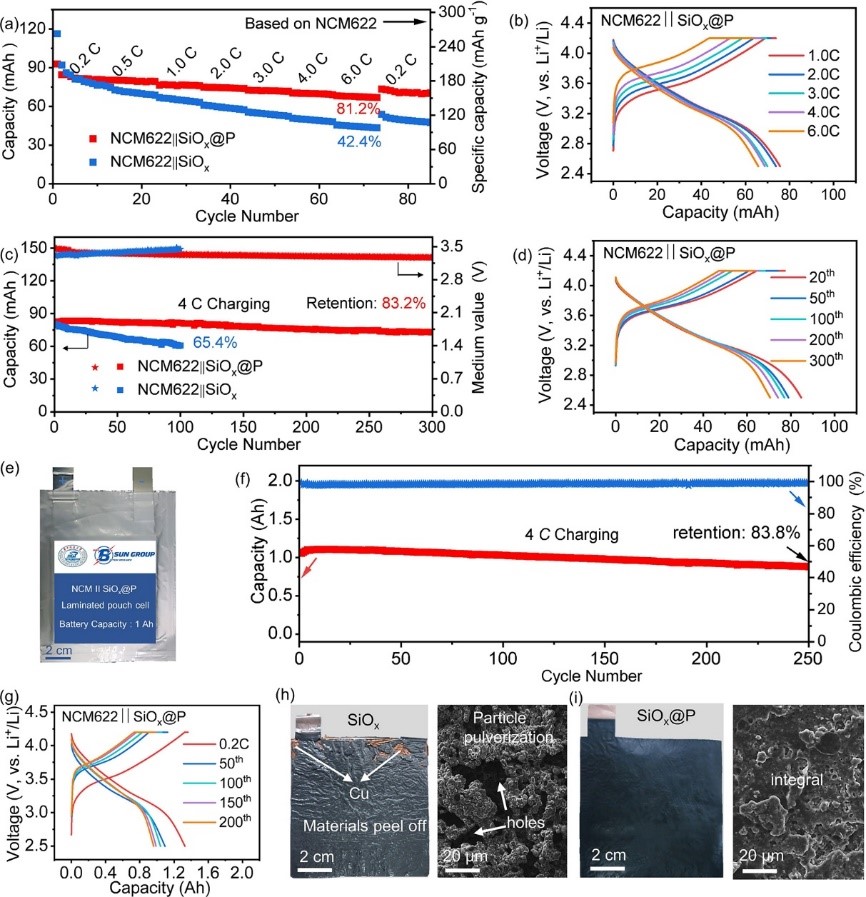
Fig. 6 (a and b) Cycling performance of the pouch cells with different SiOx anodes (a), and the charging/discharging profiles of the pouch cell with SiOx@P anode under different charging rates (0.2 to 6 C) (b). (c and d) Cycling performance of the pouch cells with different SiOx anodes (c), and the charging/discharging profiles of the pouch cell with SiOx@P anode at a charging rate of 4 C (d). (e) Photograph of the NCM622||SiOx@P laminated pouch cell. (f and g) Cycling performance (f) and the corresponding charging/discharging voltage profiles of the NCM622||SiOx@P laminated pouch cell at a charging rate of 4 C for different cycles (g). (h and i) The optical and top-view SEM images of bare SiOx (h) and SiOx@P anodes (i). The discharging rate for all the cells was fixed at 0.2 C.
Related work has been published on Energy & Environmental Science (https://pubs.rsc.org/en/content/articlepdf/2024/EE/D4EE00407H?page=search) on February 27, 2024, with the title Material-electrolyte interfacial interaction enabling formation of inorganic-rich solid electrolyte interphase for fast-charging Si-based lithium-ion batteries. The first unit for this research is Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, and it has been funded by the National Natural Science Foundation of China (No. 52072137).