Li-S batteries are promising candidates for next-generation energy-storage systems because of their high theoretical capacities and low cost of raw materials. Nevertheless, several problems remain to make Li-S batteries viable, which include the detrimental shuttling of polysulfide intermediates, complicated multiphase sulfur redox reactions, and the unrestrained precipitation of the discharge products (lithium sulfide, Li2S). Using the metallic polar materials, especially single metal atoms, as the sulfur host can trap polysulfide and accelerate the conversion kinetics between the solid (S, Li2S) and liquid (polysulfides) sulfur species. Notwithstanding these progresses, two major challenges still remain: (1) Most of the reported single metal atoms-based sulfur host only have low site fraction of metal atom (< 5 wt%), resulting in a limited number of active sites on its surface, which discounts overall polysulfides immobilization and transformation efficiency and fails to fulfill contributions of single metal atoms on battery performance enhancement. (2) The effect on single metal atoms on the reversible deposition/dissolution of lithium sulfides is still unclear, which significantly affects the sulfur utilization and the electrochemical cycling of Li-S batteries.
In view of such a serious situation, Yongming Sun, Jun Zhou, and co-workers report for the first time that the rational design of a monodispersed Co single-atom catalyst on conductive nitrogen-doped carbon nanosheet (CoSA-N-C) with the Co content as high as 15.3 wt% as a sulfur host material to synergistically regulate the conversion and deposition of lithium (poly)sulfides. Experimentations and theoretical calculations verify that the densely populated atomic Co-N species on the surface of CoSA-N-C function as not only lithiophilic-sulfiphilic sites to allow chemical polysulfides confinement but also electrocatalytic sites to facilitate the polysulfide conversion in both directions (the formation and the decomposition of Li2S). Importantly, the CoSA-N-C enables spatially controlled deposition of Li2S during the battery discharge process from conventional passivation layers that fully covered the conductive host to well-distributed nanoparticles on the host material, which favors high sulfur utilization. Using a CoSA-N-C-based sulfur cathode, a Li-S cell delivers a high areal capacity of 4.24 mAh cm-2 with 91.8 % capacity retention after 120 cycles at 0.2 C at S loading of 4.9 mg cm-2. It is believed that the concept of using the single atom catalyst to regulate the conversion and deposition of (poly)lithium sulfide offers new insight into the development of high-performance Li-S batteries and other electrochemical energy storage devices. The corresponding research results “Fast Conversion and Controlled Deposition of Lithium (Poly)sulfides in Lithium-Sulfur Batteries Using High-Loading Cobalt Single Atoms” are published in the journal of Energy Storage Materials (Energy Storage Materials, 2020, 30, 250-259).
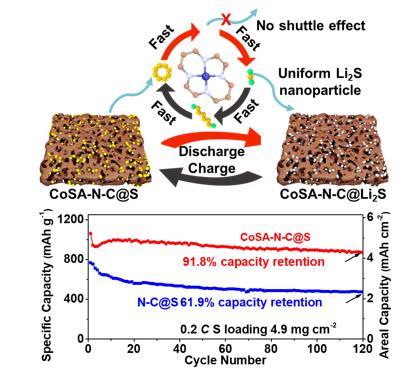
Figure. Schematic illustration of the effect of CoSA-N-C in improving the conversion kinetics of (poly)sulfides, and mediating the deposition of lithium sulfide nanoparticles.