The development of rechargeable lithium-based batteries with much higher energy and power density is of vital importance for fast expanding their applications such as electric vehicles, portable electronics, and grid energy storage. An effective approach is to search for high-capacity anode materials with low potential against cathode materials and high lithium-ion diffusion rate to replace the most widely used graphite material, which delivers a relatively low theoretical capacity of 372 mAh g-1 and slow lithium-ion diffusion rate (10-12 and 10-6 cm2 s-1). Lithium metal is a holy-grail anode due to its high theoretical specific capacity (3860 mAh g-1) and low potential (−3.040 V vs. standard hydrogen electrode). However, the practical application of lithium metal anode suffers from unsatisfactory cyclability, inferior rate capability and safety issues.
Considerable effort has been devoted to tackling the challenges of lithium metal anodes, including design of electrolyte, interface engineering, using of solid electrolytes and design of stable scaffolds/hosts. These efforts effectively alleviated certain problems of lithium metal anode mainly under moderate/low current densities (e.g., < 3 mA cm-2). However, achieving high rate capability of lithium metal anode still remains challenging. To date, there have been few significant breakthroughs that enable long-term stable cycling of lithium metal anodes at high current densities (e.g., > 5 mA cm-2) and moderately high areal capacities (e.g., > 3 mAh cm-2) with acceptable overpotentials in commercial carbonate electrolytes.
In view of current research progress, Professor Yongming Sun’s group (Huazhong University of Science and Technology) and Professor Yi Cui’s group (Stanford University) firstly investigated an interpenetrated three dimensional lithium metal/lithium tin alloy nanocomposite foil realized by a simple repeated calendering and folding process of lithium and tin foils, and spontaneous alloying reactions. Such unique nanostructure allows fast lithium diffusion over the entire electrode and enables stable lithium stripping/plating cycling under high current densities without lithium dendrite growth due to its multiple advantages: First, due to the high lithium-ion diffusion coefficient, strong lithium affinity and abundant interfaces to metallic lithium, the 3D nanostructured Li22Sn5 network forms an lithium diffusion “pathway” over the entire Li/Li22Sn5 electrode. Metallic lithium can easily transport through such “pathway” back and forth. Meanwhile, the 3D nanostructured metallic lithium and Li22Sn5 networks both provide an electron “pathway” over the entire Li/Li22Sn5 electrode. Second, the potential difference (~0.3 V) between Li22Sn5 and metallic lithium can act as the driving force for lithium diffusion through such a “pathway”. These advantages enable fast lithium diffusion over the entire the Li/Li22Sn5 electrode, leading to good rate capability. Furthermore, fast lithium diffusion helps to alleviate the formation of lithium metal dendrites during cycling41 and thus improves the safety of rechargeable lithium metal batteries. Third, the Li22Sn5 is less reactive with the liquid electrolyte than the metallic lithium due to its higher chemical potential. Thus, less electrolyte consumption and longer cycle life can be expected. Fourth, the 3D interconnected Li22Sn5 network remains invariant in the composition and structure on cycling and is capable of working as a stable host for stripping/plating of lithium metal and thus addressing the challenge of significant volume change. The author demonstrate that a lithium/lithium tin alloy foil electrode sustains stable lithium stripping/plating under 30 mA cm-2 and 5 mAh cm-2 with a very low overpotential of 20 mV for 200 cycles in a commercial carbonate electrolyte. Cycled under 6 C (6.6 mA cm-2), a 1.0 mAh cm-2 LiNi0.6Co0.2Mn0.2O2 electrode maintains a substantial 74% of its capacity by pairing with such anode.
This research work provides a new direction for the design of high-rate lithium metal anode materials. Related work has been published on Nature Communications (DOI: 10.1038/s41467-020-14550-3) on February 11, 2020, with the title of “Mechanical rolling formation of interpenetrated lithium metal/lithium tin alloy foil for ultrahigh-rate battery anode".
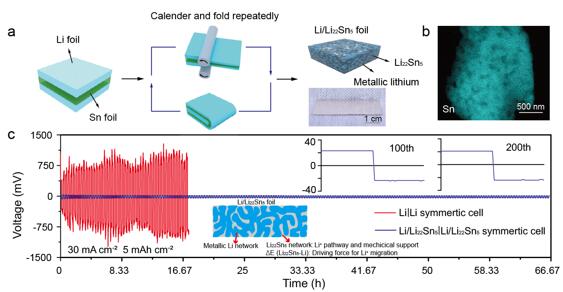
(a) Schematic of the fabrication of the Li/Li22Sn5 nanocomposite foil. (b) X-Ray (EDX) elemental mapping image for Sn in 3D Li22Sn5 framework. (c) Galvanostatic lithium plating/stripping cycling and voltage profiles of the Li/Li22Sn5| Li/Li22Sn5 and Li|Li symmetric cells.