The unprecedented development of technologies, including electric vehicles, portable electronics, and grid energy storage, calls for efficient energy storage devices with low cost, long lifespan and high safety. Rechargeable batteries employing Zn metal anode and aqueous electrolyte are emerging as strong candidates due to their cost-effectiveness, high safety, environmental friendliness, and considerable energy density. Particularly, Zn metal offers a number of advantages as a battery anode, including high theoretical capacity (820 mAh/g), low redox potential (-0.76 V vs. standard hydrogen electrode (SHE)) and high abundance. However, it remains a great challenge to achieve high-performance Zn metal anode with high stability and electrochemical reversibility due to the severe problems such as Zn dendrite growth and corrosion. To improve the electrochemical reversibility of Zn metal anode, several important strategies, including employing stable metallic Zn host/substrate, using protective surface layer, 3D structured metallic Zn, electrolyte engineering, as well as construction of in-situ solid electrolyte interphase have been proposed and exciting progresses have been achieved. However, a significant challenge, corrosion of Zn metal anode in aqueous electrolyte, still remains, which causes the instability of Zn metal electrode during the battery resting and cycling, and inhibits its practical application in rechargeable aqueous batteries.
In view of the current research process, Professor Yongming Sun’s group (from Huazhong University of Science and Technology) firstly investigated the corrosion of Zn metal electrode in a typical mild acidic ZnSO4 aqueous electrolyte. They uncovered that the corrosion of Zn metal in 3M ZnSO4 electrolyte started from the surface accessible to the electrolyte, where Zn4(OH)6SO4•5H2O microsheets were first formed, accompanying with the evolution of hydrogen gas, resulting in significant corrosion of Zn metallic electrode. It should be noted that the corrosion of Zn metal was not uniform. The deepest corrosion pit reached 132.2 μm after a dwell time of 30 days. The as-formed deactivated Zn side product, gas evolution, and consumption of electrolyte would increase electrochemical impedance of Zn metal anode and lead to battery degradation. Given that, they introduced chemically inert metallic Cu to improve the anti-corrosive property of Zn metal electrode. A simple replacement reaction was utilized to construct a uniform Cu/Zn composite with dense structure, which was electrochemically converted to Cu-Zn nanoalloy/Zn hybrid during battery cycling. As expected, such Cu-Zn/Zn electrode displayed much better stability and electrochemical reversibility than the pristine Zn metal anode. A Cu-Zn/Zn||Cu-Zn/Zn symmetric cell showed stable electrochemical cycling of 1500 cycles at 1 mA/cm2 with a fixed capacity of 0.5 mAh/cm2 with little change in overpotential (46 mV) after a dwell time of 30 days, suggesting the remarkable anti-corrosion property and electrochemical reversibility of the Cu-Zn/Zn electrode. In contrast, the Zn||Zn symmetric cell failed and could not cycle after a dwell time of 30 days. Moreover, they further prepared MnO2 on carbon cloth (MnO2@CC) electrode and used it to pair with Cu/Zn electrode to assemble full cells and evaluate the practicability of the Cu/Zn anode. The bare Zn||MnO2 full cell maintained only 27.6% of its initial capacity after 300 cycles at a rate of 10 C (1C = 308 mA/g, based on MnO2). Meanwhile, the Cu/Zn||MnO2 full cell exhibited excellent cycling stability, which delivered 94.2% of its initial capacity after 500 cycles. Such robust electrochemical performance supports the practical application of Cu/Zn electrode in rechargeable aqueous batteries.
The researchers believe that this work provides fundamental understanding of the corrosion of Zn metal electrode in aqueous batteries, shows the important influence of corrosion on electrochemical performance of Zn metal electrode, and sheds light on the rational design of anti-corrosion Zn anode for stable and deeply rechargeable aqueous batteries. Related work has been published on Energy Storage Materials (Energy Storage Mater., 2020, https://doi.org/10.1016/j.ensm.2020.01.032) On February 5, 2020, with the title of “Chemically resistant Cu-Zn/Zn composite anode for long cycling aqueous batteries".
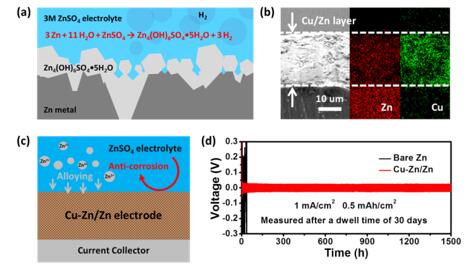
(a) Schematic illustration of the chemical corrosion of Zn metal electrode in 3M ZnSO4 electrolyte; (b) Cross-section SEM and EDS elemental mapping images of the Cu/Zn electrode; (c) Schematic illustration of the function of the Cu-Zn alloy on Cu-Zn/Zn electrode; (d) Voltage profiles of Zn||Zn and Cu-Zn/Zn||Cu-Zn/Zn symmetric cell at 1 mA/cm2 and 0.5 mAh/cm2 after a dwell time of 30 days.