Li-S batteries are promising candidates for the next-generation energy-storage systems because of their high theoretical capacities and low cost of raw materials. Nevertheless, several problems remain to make Li-S batteries viable, which include the notorious shuttling of soluble lithium polysulfide (LPS) intermediates with sluggish redox reactions and uncontrolled precipitation behavior of the charge/discharge products. Introducing polar inorganic materials into sulfur cathode as both the immobilizer and catalyst of LPS can overcome the above issues to some extent. However, several issues still exist: (1) Most of the reported sulfur cathodes possessed a large proportion (>15 wt.%) of these electrochemically inert materials, which significantly decreased the practical energy density of Li-S batteries. (2) These polar inorganic materials usually exhibited inferior electrical conductivity and impeded the electron migration during the charge/discharge process, resulting in a poor cycling performance and rate capability. (3) Little attention has been paid to regulating the deposition of insulating lithium sulfides on these matrixes, which plays an important role in realizing high sulfur utilization. In view of such a serious situation, Prof. Yongming Sun and co-workers reported a semi-liquid cathode composed of an active LPS solution/carbon nanofiber (CNF) composite layer capped with a carbon nanotube (CNT) thin film decorated with metallic Mo nanoclusters (CNF/LPS/Mo/CNT) that regulate the electrochemical redox reactions of LPS. The trace amount (0.05 mg cm-2) of metallic Mo on the CNT film provides sufficient capturing centers for the chemical immobilization of LPS. Together with physical blocking of LPS by the compact CNT film, free-diffusion of LPS is significantly restrained and the self-discharge behavior of the Li-S cell is thus effectively suppressed. Importantly, the metallic Mo nanoclusters enable fast catalytic conversion of LPS and regular deposition of lithium sulfide. As a result, the engineered cathode exhibits a high reversible areal capacity of 4.75 mAh cm-2 after 100 cycles at 0.2 C for a cathode with a high sulfur loading of 7.64 mg cm-2. This work provides significant insight into the structural and materials design of an advanced sulfur-based cathode that effectively regulates the electrochemical reactions of sulfur species in high-energy Li-S batteries. The relative work has been published in ACS NANO (https://pubs.acs.org/doi/10.1021/acsnano.9b09135) with the title of “Enhanced Chemical Immobilization and Catalytic Conversion of Polysulfide Intermediates Using Metallic Mo Nanoclusters for High-Performance Li-S Batteries”. The first author is Yuanjian Li and the corresponding author is Prof. Yongming Sun.
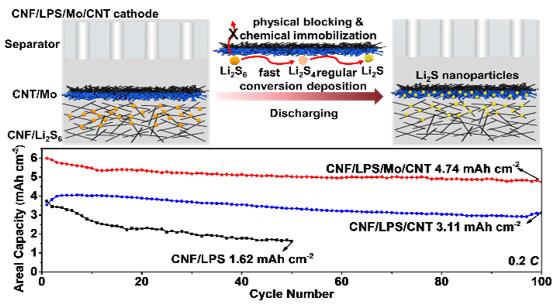
(a) Schematic illustration of the effectiveness of the designed CNF/LPS/Mo/CNT cathode in addressing the free diffusion of LPS, catalyzing their fast conversion and realizing regular deposition of lithium sulfides (Li2S) nanoparticle during discharge. (b) The electrochemical performance of the designed CNF/LPS/Mo/CNT cathode, achieving a high areal capacity of 4.75 mA h cm-2 after 100 cycles at 0.2 C with a high sulfur mass loading of 7.64 mg cm-2.