On February 4, 2019, the team of Prof. Zhihong Zhang from Wuhan National Laboratory for Optoelectronics at the Huazhong University of Science and Technology, published online their latest paper in Nature Communications entitled “Immune modulation of liver sinusoidal endothelial cells by melittin nanoparticles suppresses liver metastasis”. Their work is the first to achieve the targeted modulation of liver sinusoidal endothelial cells and provide a new strategy to suppress liver metastasis.
As a digestive organ, liver is well known for its metabolism, detoxification and endocrine functions. In recent years, more and more studies have confirmed that the liver is an immune organ which contains unique functional endothelial cells and immune cell subsets. The liver sinusoidal endothelial cells (LSEC) are liver tissue-specific vascular endothelial cells which are not only the main components of hepatic sinusoidal wall but also widely involved in various physiological functions of the liver (immune regulation, liver regeneration, liver metabolism, etc) and pathological processes (liver fibrosis, cirrhosis, etc). Therefore, targeted modulation of LSEC has potential value for improving the involved processes. At present, however, there is no effective approach to target LSEC in vivo, which limits the application research on specific regulation of LSEC. Focusing on the problem, Prof. Zhihong Zhang’ research team established a new method for targeted labeling and immune modulation of LSEC. Using this strategy, they found that it could specifically activate LSEC to change the immune microenvironment in the liver, and successfully block liver metastasis of various tumors.
It was found that melittin lipid nanoparticles (α-melittin-np) containing RKR polypeptide sequences could effectively target LSEC and induce immune activation after intravenous injection in vivo. Intravital imaging showed that LSECs fluoresced within 20 s after intravenous injection of fluorescent labeled α-melittin-NPs, and the high-efficiency targeted labeling effect was maintained for up to 12 h. LSEC transcriptome sequencing results showed that the mRNA level of immune-related molecules significantly increased, and the GO enrichment analysis showed that the differentially expressed genes (DGE) were mainly involved in immune cell chemotaxis and lymphocyte activation. Combined with flow cytometry and liquid chip assay, they found that the co-stimulatory molecules (CD80, CD86, MHC-Ⅱ) were highly expressed on LSEC, and the levels of cytokines (IL-1α) and chemokines (Cxcl9, Cxcl10, etc) increased in liver along with increased immune cell infiltration and NK cell activation. Thus, α-melittin-NPs break the LSEC-mediated immune tolerance state by selectively activating LSEC, eventually leading to suppression of the experimental liver metastasis of various tumors (melanoma, breast cancer, colon cancer) and spontaneous liver metastasis of breast cancer.
This work was supported by the National Key Research and Development Program of China (2017YFA0700403), the National Science Fund for Distinguished Young Scholars (81625012), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (Grant No. 61721092), and the Major Research plan of the National Natural Science Foundation of China (Grant No. 91442201).Prof. Zhihong Zhang and Prof. Qingming Luo are co-corresponding authors of this paper, and doctoral candidate Xiang Yu is the first author. As co-authors, Lu Chen, Jianqian Liu, Bolei Dai and Guoqiang Xu participated in the related work, and Prof. Guanxin Shen provided guidance on this work.
Link to the paper: https://www.nature.com/articles/s41467-019-08538-x
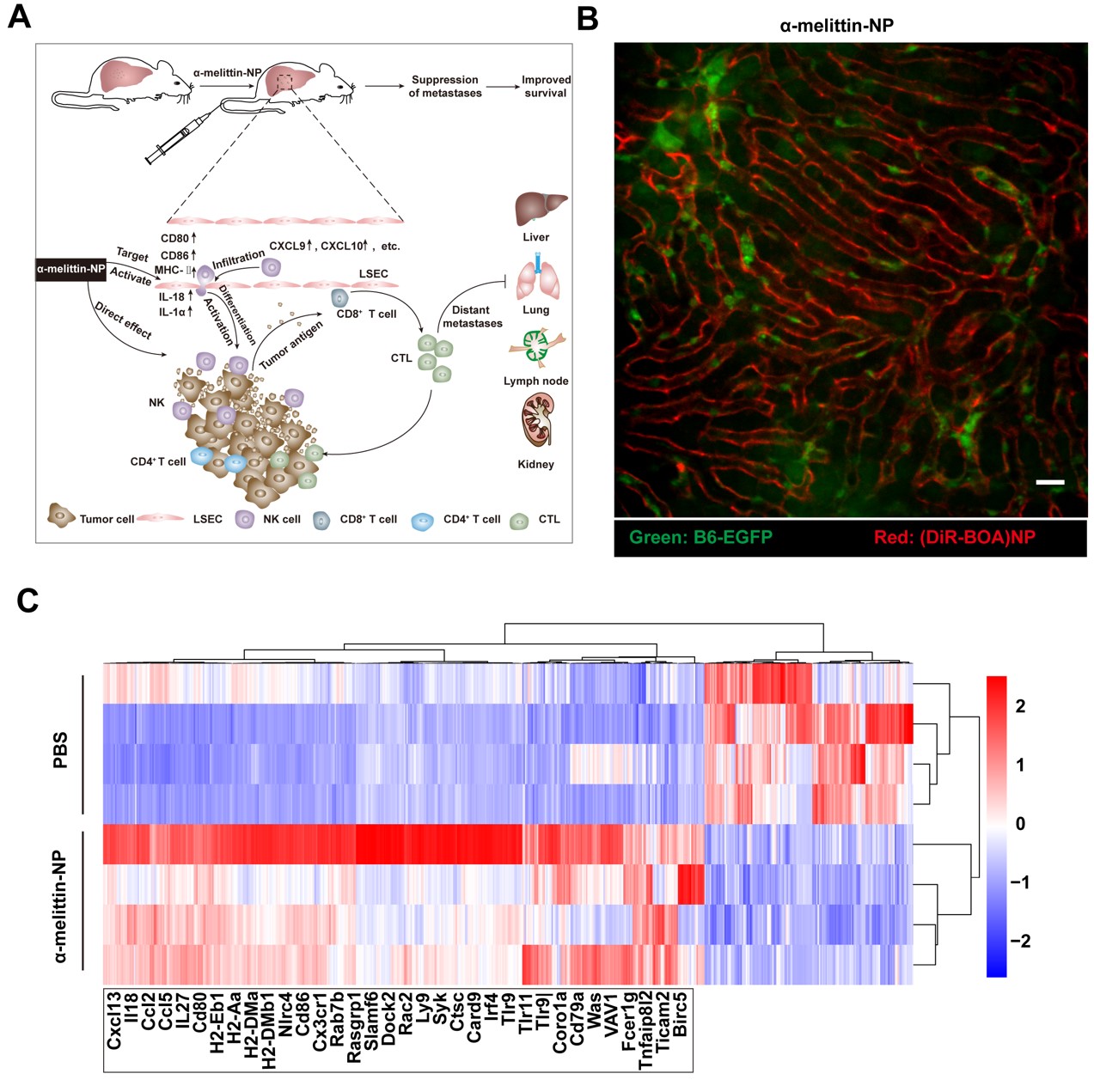
Fig. Targeted labeling and immune modulation of LSEC. (A) Schematic description of a plausible mechanism for α-melittin-NP-mediated suppression of liver metastasis. (B) Intravital imaging of targeting ability of α-melittin-NPs to LSEC. Scale bar, 20 μm. (C) Heatmap of the 452 upregulated and 157 downregulated genes in the α-melittin-NP-treated LSECs. Some representative genes related to immune response are showed below.