Sn metal possesses high lithium (Li) storage capacity (990 mAh g-1), average lithiation potential of ~0.4 V (vs. Li/Li+), and good electrical conductivity (9.1×10^6m·Ω), thus is a promising anode for high-energy-density and fast-charging LIBs. However, Sn-based anodes suffer from drastic volume changes(up to260%for full lithiation from Sn to Li4.4Sn)and stress during the charge/discharge processes, resulting in the pulverization of the materials and rapid capacity degradation. Till now, several successful strategies have been developed to address the above issues at the particle level, including preparing Sn nanoparticles, embedding Sn nanoparticles into carbon matrix, and designing Sn-based alloy nanoparticles. However, the applications of nanoparticle electrodes are limited by their intrinsic disadvantages, including complex fabrication procedures, low tap density, low volumetric energy density and high electrolyte uptake. Compared to Sn nanoparticle electrodes, Sn foil has greater advantages in terms of processability and volumetric energy density. Nevertheless, the huge local stresses and volumetric strains generated when the Sn foil undergoes uneven alloying reactions with lithium, leading to the chemical-mechanical failure behavior of the Sn foil during cycling. The key to solving these problems lies in improving the electrolyte wettability of the Sn foil, mitigating the grain boundary effect and volume expansion of the Sn foil.
Yongming Sun’ group systematically analyzed the failure process of Sn foil electrode during cycling through a series of chemical-mechanical simulations and electrochemical tests, and proved that the chemical-mechanical failure behavior of Sn foil can be effectively mitigated by grain refinement and pore design. Furthermore, the authors designed a three-dimensional interconnected porous Sn (3DIP-Sn) foil with excellent electrolyte wettability, refined grains (300-500 nm), and moderate porosity (58.7%), which can effectively promote uniform lithiation and stress relief of the Sn foil. As a result, the 3DIP-Sn foil exhibits ultralong cycle life of 4400 h (1000 h for pristine Sn foil) at 1 mAh cm-2and 0.5 mA cm-2. Impressively, 3DIP-Sn||LiFePO4(LiFePO4, loading ~7.1 mg cm-2) full-cell has 80% capacity retention rate after 500 cycles, and 3DIP-Sn||NCM622 (NCM622, loading ~18.4 mg cm-2) full-cell also exhibits a good cycling stability.

Figure 1.(a, b)Schematic illustrations of the electrochemicallithiationprocesses ofSn foils with different structures.Chemo-mechanical simulated time series snapshots of (c) Li and (d) effective stress distributions in Sn foils with large-sized grain, small-sized grain, and small-sized grain and porous structures, respectively.
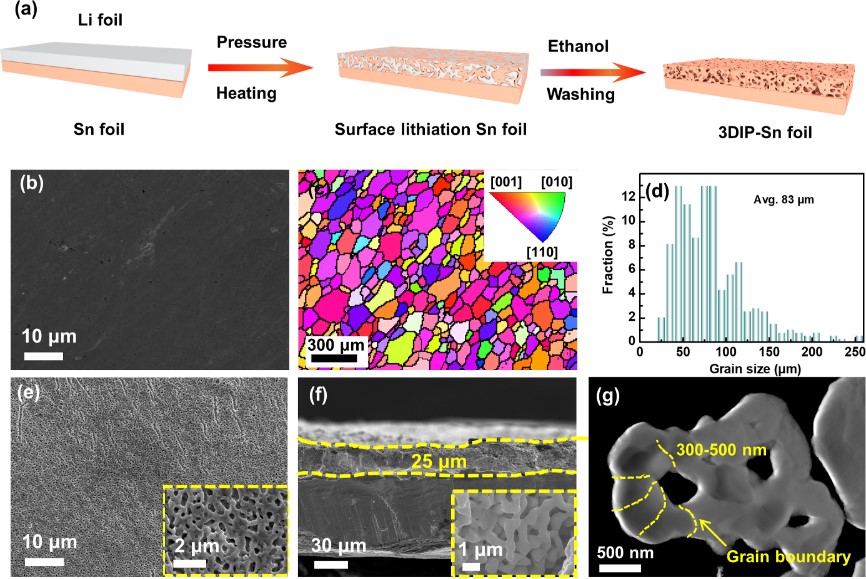
Figure 2.(a) Schematic illustration of the fabrication process of the 3DIP-Sn foil. (b) Top-viewSEM image, (c) the electron-backscatter diffraction (EBSD)inversepole figure map, and (d) the grain size distribution of the pristine Sn foil. (e) Top-view,(f)cross-section SEM, and (g) HAADF images of the 3DIP-Sn foil.The insetsin (e, f) showedtheenlarged SEM imagesfor the3DIP-Sn foil.
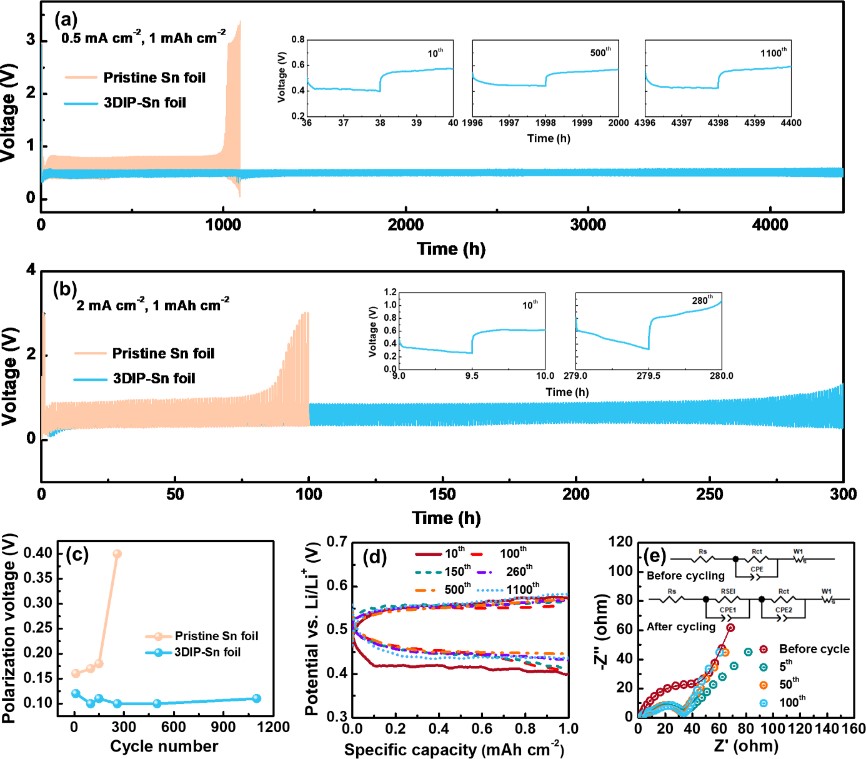
Figure 3.Galvanostatic cycling performance ofthepristine Sn and 3DIP-Sn foil electrodes in half cells using Li metal as the counter electrode at(a)0.5 mA cm-2and1mAh cm-2,and (b)0.5 mA cm-2and1mAh cm-2. (c) Potential hysteresis of the pristine Sn and 3DIP-Sn foil electrodes at different cycles at0.5 mA cm-2and 1 mAh cm-2.(d) Voltage profiles of the 3DIP-Sn foil electrode at different cycles at 0.5 mA cm-2and 1 mAh cm-2. (e)EIS spectraofthe3DIP-Sn foil electrode after different cycles.The inset shows the corresponding fitted equivalent circuit diagrams.

Figure 4.Electrochemicalcycling performanceof thepristineSn||LiFePO4and3DIP-Sn||LiFePO4 cells with LiFePO4 loadings of (a) 1.7 mg cm-2and (b) 7.1 mg cm-2, respectively. (c) Cycling performance of the pristine Sn||NCM622and 3DIP-Sn||NCM622 cells with NCM622 loading of ~18.4 mg cm-2. (d) Rate capability of the pristine Sn||LiFePO4 and 3DIPA-Sn||LiFePO4 cells at various current densities with LiFePO4 loading of ~1.7 mg cm-2, and (e) the corresponding galvanostatic charge/discharge profiles for the 3DIP-Sn||LiFePO4cells. (f) Rate capabilityof the pristine Sn||LiFePO4 and 3DIP-Sn||LiFePO4cells at various current densities with a LiFePO4loading of ~7.1 mg cm-2.
Related work has been published onJournal of Energy Chemistry(https://doi.org/10.1016/j.jechem.2021.03.053) on April 09, 2021 with the title of “Circumventing chemo-mechanical failure of Sn foil battery anode by grain refinement and elaborate porosity design”. It has been funded by National Natural Science Foundation of China (No. 520721137, 51802105).