An extensive dependence on fossil fuels and rising energy demands is driving people to develop the energy economy in a more clean and sustainable way. Rechargeable metal batteries with cost-effectiveness, high safety, and long life span are promising for grid-scale storage of renewable energy (e.g., solar, wind, and tidal) as well as for applications in electric vehicles. Metal foils, including metallic zinc (Zn) and metallic lithium (Li) foils, are the most commonly studied/used metal electrode forms for rechargeable metal batteries due to their scalable preparation and easy processing. For example, metallic Zn foil electrode, with its abundant resource of raw materials, ecological friendliness, and high theoretical capacity (i.e., 5854 mAh cm-3and 820 mAh g-1), is promising for rechargeable batteries employing mild aqueous electrolyte with high safety. However, it encounters inherent limitations(including poor chemical stability and electrochemical reversibility)for practical applications.
In view of the current research process, Professor Yongming Sun's (Huazhong University of Science and Technology) and Prof. Yi Cui (Stanford University) employed a facile replacement reaction to fabricate a three-dimensional (3D) interdigitated metal/solid electrolyte composite electrode, which not only provides a stable host structure for buffering the volume change within the composite but also prevents side reactions by avoiding the direct contact between active metal and liquid electrolyte, thus improvesthe electrochemical performance of Zn metal electrode in aqueous batteries.
Replacement reaction is one of the four elementary chemical reactions,thereplacement of polycrystallineZn metal foilwith InCl3initiates on the surface, and it penetrates deeplyalong the grain boundaries to the bulkand aninterdigitated Zn/In architecture was fabricated. After electrochemicalactivation in aqueous ZnSO4 electrolyte, In metal was in situ oxidized to hydroxide sulfates solid electrolyte, and the initial Zn/In architecture transformed to the Zn/IHS composite electrode. The IHS displayed a compact structureconsisting of ultrafine nanoparticles, whichfacilitate the diffusion of Zn2+ions and protect metallic Zn from sidereactions.Meanwhile,the 3Dinterdigitation of the IHS solid electrolyte benefited thespecific surface area and thus the efficient use of the active Znmetal. As a result,the as-achieved Zn/HIS electrode displayed cycle-stable performance withlow overpotential of 10 mV under ultrahigh current density and areal capacity (20 mA cm-2, 20 mAh cm-2), which outperformed allthe reported Zn metal electrodes in mild aqueous electrolyte. Moreover,theZn/IHS||MnO2 full cell delivered 93.9% of its initial capacity(i.e.,100 mAh g-1) after 1000 cycles under 10 C. As contrast, the bare Zn||MnO2 full cell retained only 53.6% of its initial capacity under the sametest condition.
The researchers believe that the above demonstration of 3D interdigitatedZn/IHS composite structure offered a new perspective fordesigning an advanced Zn electrode with high mechanical stability, suppressed side reactions, and good reversibility forrechargeable aqueous batteries, which opens a new door forrealizing the targeted development of advanced aqueous Znmetal batteries. Related work has been published on Journal of the American Chemical Society(J. Am. Chem. Soc.2021,https://pubs.acs.org/doi/abs/10.1021/jacs.0c11753) On February 17, 2021, with the title of "A Replacement Reaction Enabled Interdigitated Metal/SolidElectrolyte Architecture for Battery Cycling at 20 mA cm-2and 20mAh cm-2".
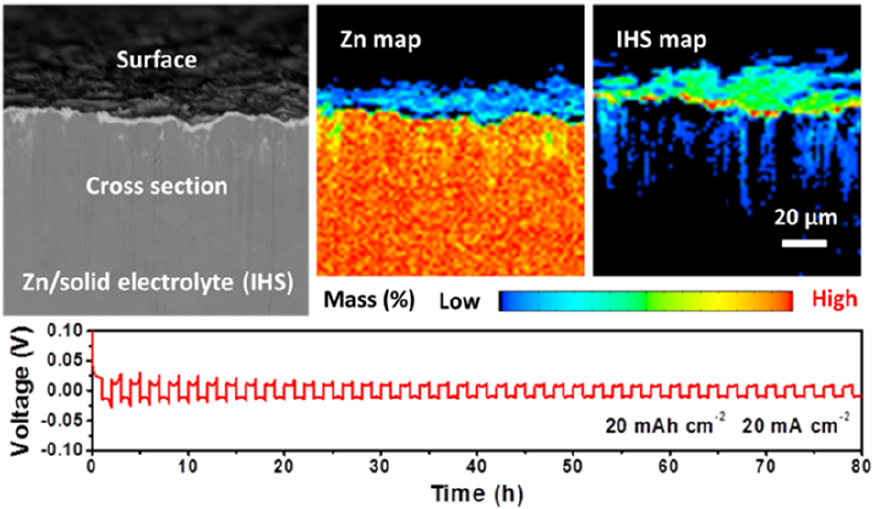
Fabrication and electrochemical performance of 3D interdigitated Zn/IHS electrode