Metallic Li possesses the highest theoretical specifc capacity(3860 mA h g-1) and lowest electrochemical potential (-3.04 Vversus standard hydrogen electrode) among anodes, and is theultimate anode choice for rechargeable batteries.However, its practicalapplication is still impeded by severalserious issues including uncontrolled Liplating/stripping behavior and undesiredside reactions at the anode side due tolarge volume change and high reactivityof metallic Li during the stripping/platingcycling.The design of Limetal electrode with uniform SEI and stable stripping/platingbehavior is of vital importance for practical LMBs.Till now, numerous strategies have been explored to improveLi metal electrodes, including surface protection layer, 3D host,solid electrolyte, and electrolyte engineering.Among thevarious strategies, using electrolyte additives is considered asone of the most facile and effective approaches. Additivesin electrolyte can effectively regulate metallic Li depositionby the optimization of the solvation structures of Li+and thereinforcement of physical and chemical properties of SEI.
Yongming Sun'group proposed a concept of "salt-in-metal" and aLi/LiNO3composite foil is constructed such that a classic electrolyte additive, LiNO3, is embedded successfully into the bulk structure of metallic Liby a facile mechanical kneading approach. The LiNO3reacts with metallicLi to generate Li+conductive species (e.g., Li3N and LiNxOy) over the entireelectrode.These derivatives afford a stable solid electrolyte interphase (SEI)and effectively regulate the uniformity of the nucleation/growth of Li on initialplating, featuring a low nucleation energy barrier and large crystalline sizewithout mossy morphology. Importantly, these derivatives combined withLiNO3can in-situ repair the damaged SEI from the large volume changeduring Li plating/stripping, enabling a stable electrode-electrolyte interfaceand suppressing side reactions between metallic Li and electrolyte. Stablecycling with a high capacity retention of 93.1% after 100 cycles is obtained forfull cells consisting of high-loading LiCoO2cathode (≈20 mg cm-2) and composite metallic Li anode with 25 wt% LiNO3 under a lean electrolyte condition(≈12 µL) at 0.5 C.Thiswork not only presents a new strategy for the introduction ofSEI stabilizer with low solubility in electrolyte solvents, but alsoextends the consequent protective structure from the surface tothe bulk of metallic Li electrode, enhancing its electrochemicalperformance.

Figure 1.(a) The fabrication of LLNO composite. The evolution of (b) pure Li and (c) LLNO composite electrodes during electrochemical cycling.
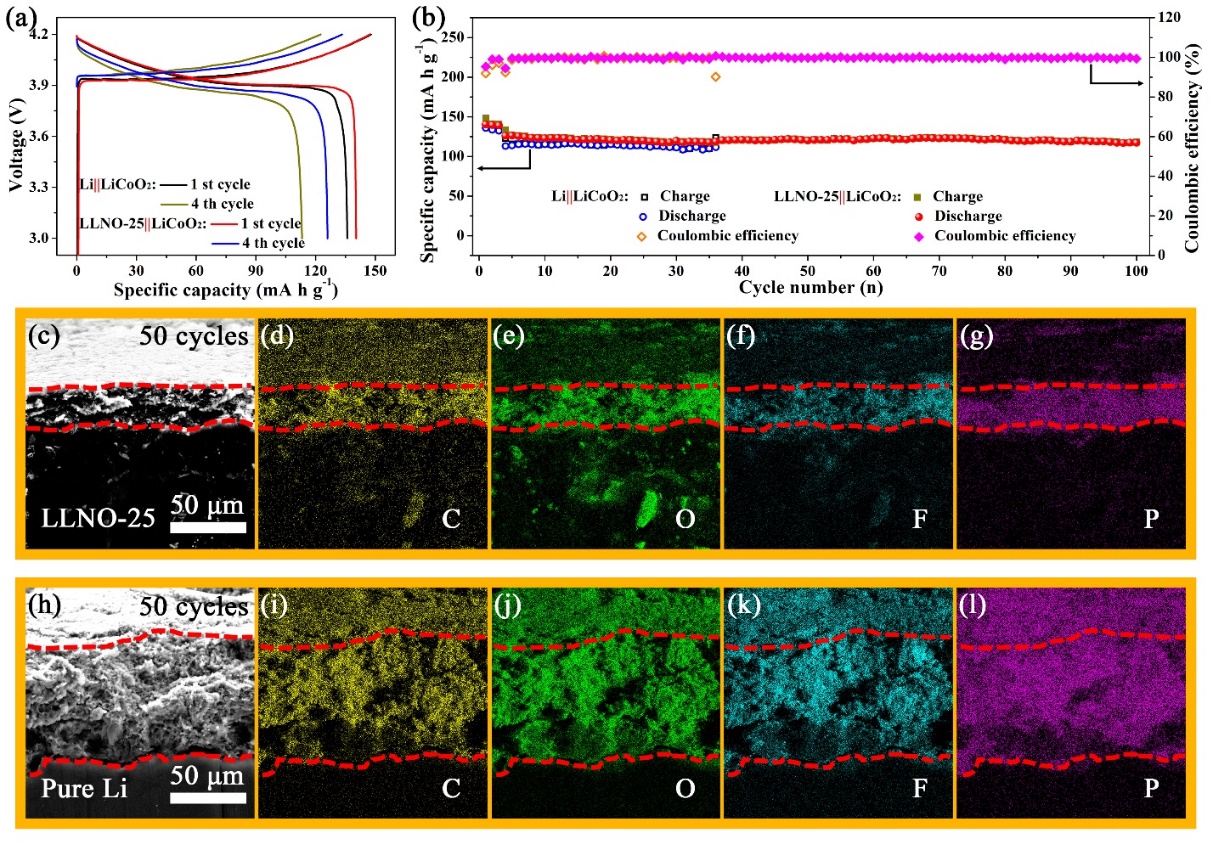
Figure 2.Electrochemical performances of the pure Li and LLNO-25 composite anode coupled with high-loading commercial LiCoO2cathode (~20 mg cm-2) at a current density of 0.1C(the first three cycles) and 0.5 C (the following cycles). (a) Voltage profiles of the LLNO-25|| LiCoO2and LiCoO2||Li cells and (b) their corresponding cycling performance. (c) Cross-sectional SEM image of a LLNO-25 composite anode after 50 cycles and (d-g) the corresponding EDS mapping images. (h) Cross-sectional SEM image of a pure Li anode after 50 cycles and (i-j) the corresponding EDS mapping images.
Related work has been published onAdvanced Functional Materials(Adv. Funct. Mater., 2021,https://onlinelibrary.wiley.com/doi/10.1002/adfm.202010602) On March 3, 2021, with the title of "A Salt-in-Metal Anode: Stabilizing the Solid ElectrolyteInterphase to Enable Prolonged Battery Cycling ". The first unit for this research is Wuhan National Laboratory for OptoelectronicsHuazhong University of Science and Technology, and it has been funded by National Natural Science Foundation of China (No. 52002136, 51802105).