Metallic sodium (Na) has been regarded as one of the most attractive anodesfor Na-based rechargeable batteries due to its high specific capacity, low working potential, and high natural abundance. However, several important issues hinder the practicalapplication of the metallic Na anode, including its high reactivity with electrolytes,uncontrolled dendrite growth, and poor processability. Until now, several important publications have taken the directions of electrode surface coating, construction of an artificial SEI film, composite Na metal anode, solid electrolyte, as well as the optimization of electrolyte composite to improve the electrochemical performance of Na metal anodes. Among all of the strategies, adding solid electrolyte interface (SEI) stabilizers into electrolyte to form a stable SEI to protect metallic Na is the most facial and efficiency strategies. However, the application of most SEI stabilizers was significantly restricted by the low solubilityintheelectrolyte.
Yongming Sun'group have developed a Na/NaNO3composite foil featured with NaNO3 SEI stabilizers uniformly embedded within the metallic Na bulk by a mechanical kneading method. In this composite structure, NaNO3was in situ reduced and was involved in the formation of a robust NaNxOy and Na3N species-rich SEI, which can homogenize Na+ flow and accelerate their diffusion through the SEI due to the enhanced conductivity, and decrease the side reactions between the active metallic Na and electrolyte. Moreover, due to the uniform implantation of NaNO3 and its reductive products within the metallic Na bulk, the processability of the as-fabricated Na/NaNO3 composite foil was significantly improved. As expected, stable cycling of 600 h with low voltage polarization was achieved for the Na/NaNO3 electrodein a symmetric cell configuration at 0.5 mA cm-2and 0.5 mAhcm-2 in a carbonate-based electrolyte. Furthermore, aNa/NaNO3||Na3V2(PO4)2O2F (NVPOF) cell with a Na anodeareal capacity loading of ∼5 mAh cm-2 sustained stable cyclingfor over 180 cycles, while theNa||NVPOFcell with the bareNa anode displayed fast capacity fade after 70 cycles.
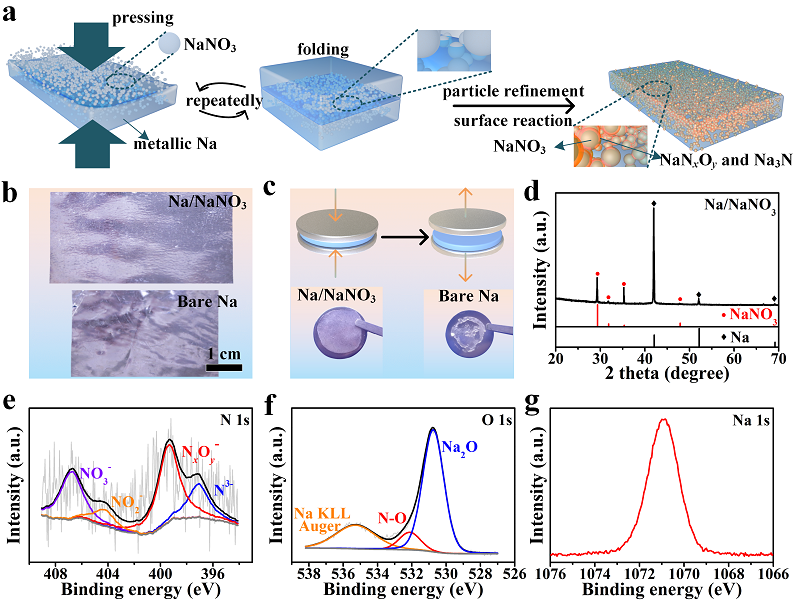
Figure 1.(a) Schematic diagram of the fabrication of Na/NaNO3 composite foil using a mechanical kneading approach. Repeated folding and calendering operations are adopted to fabricate the Na/NaNO3 composite foil. (b) Digital photos of the Na/NaNO3 composite foil and the pure metallic Na foil with same thickness of 400 μm and (c) after the release from an external mechanical pressure of 10 MPa in a coin cell. (d) XRD results of the Na/NaNO3 composite foil, corresponding to NaNO3(JCPDS#36-1474) and pure metallic Na (JCPDS#22-0948). High-resolution XPS spectra of (e) N 1s, (f) O 1s and (g) Na 1s for the Na/NaNO3 composite foil.
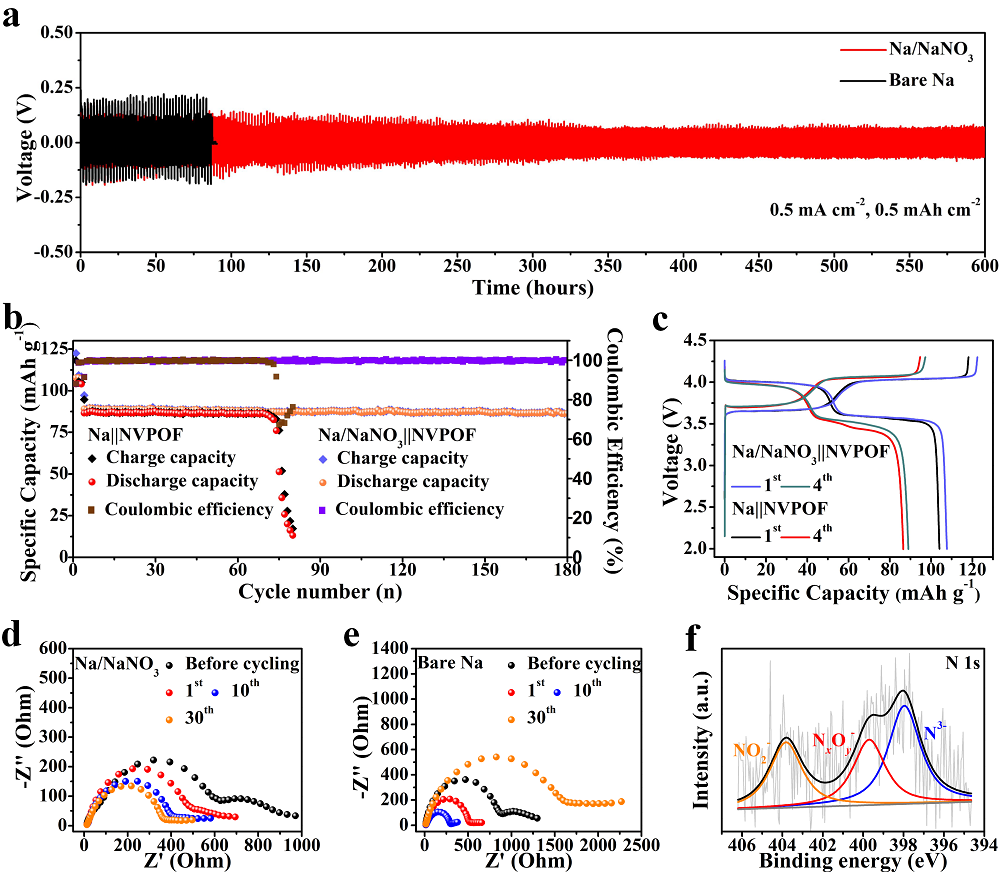
Figure 2.Electrochemical performance of the Na/NaNO3and metallic Na electrodes. (a) Voltage profiles of the Na/NaNO3||Na/NaNO3and Na||Na symmetric cells cycled at 0.5 mA cm-2and 0.5 mAh cm-2. (b) Cycling performance of Na/NaNO3||NVPOF and Na||NVPOF cells and (c) the corresponding charge/discharge profiles after the 1stand 4thcycles. The cells were cycled at 0.5 C (1 C = 130 mA g-1) for the initial 3 cycles followed by cycling at 5 C at a voltage range from 2.0 to 4.3 V. (d, e) Nyquist plots of the Na/NaNO3||Na/NaNO3and Na||Na symmetric cells after different cycles and (f) high-resolution N 1s spectrum of the Na/NaNO3 electrode after 50 cycles at 1 mA cm-2and 1 mAh cm-2.
Related work has been published on ACS Applied Materials & Interfaces(https://pubs.acs.org/doi/full/10.1021/acsami.1c01571) on March 12, 2021 with the title of "Addressing the Low Solubility of a Solid Electrolyte Interphase Stabilizer in an Electrolyte by Composite Battery Anode Design". The first unit for this research is Wuhan National Laboratory for Optoelectronics Huazhong University of Science and Technology, and it has been funded by National Natural Science Foundation of China (No. 520721137, 51802105).