Due to high theoretical capacity (4200 mAh g-1), low cost and abundance in resources,Silicon (Si)-based material is a promising anode material for next-generation lithium-ion batteries (LIBs). Unfortunately,the commercial application of Si-based materials is hindered by several severe issues, including lowelectrical conductivity, dramatic volume change (>300%), and unstable solid electrolyte interphase (SEI).Silicon sub-oxides (SiOx) showoutstanding virtueofless volume expansion/shrinkagein comparison to Si, and cansuppress the undesirable side reactionsand improve the electrochemical cycling stability.However, to date there lacks a facile and mild approach to regulate the oxygen content in SiOxduring the materials fabrication.A low content of oxygen cannot guarantee the effective suppression of volume change while a high content of oxygen would result in severe capacity reduction and large voltage hysteresis in voltage curves.
Yongming Sun'group reported the fabrication of a silicon oxide-carbon (SiOx/C) nanocomposite through the reaction between silicon particles with fresh surface and H2O in a mild hydrothermal condition, as well as conducting carbon coating synchronously. Generally, regular Si particles with native oxidation layer tended to maintain its component and structure even at high hydrothermal temperature (200 °C) due to the protection from the surficial passivation layer, and controllable oxidation was only realized for Si particles to produce uniform SiOx after the removal of the passivation layer. The uniform oxidation and conductive coating offer the as-fabricated SiOx/C composite good stability at both particle and electrode level over the long-term electrochemical cycling.The as-fabricated SiOx/C composite delivered a high reversible capacity of 1133 mAh g-1at0.5A g-1 with 89.1% capacity retention after 200 cycles. With 15 wt% SiOx/C composite, graphite-SiOx/C hybrid electrode displayed a high reversible specific capacity of 496 mAh g-1and stable electrochemical cycling with a capacity retention of 90.1% for 100 cycles.
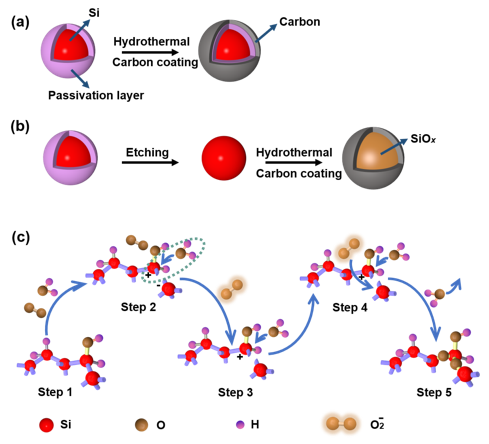
Figure 1.(a) Dense passivation layer on the Si surface, which prevents its further oxidation.(b) Schematic illustration of controllable oxidation of Si with fresh surface and synchronous carbon coating. (c) Schematic of the controllable oxidation under hydrothermal condition for Si particles with a fresh surface.
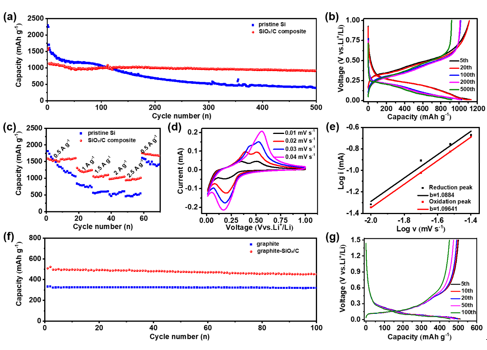
Figure2.The electrochemical performance of the SiOx/C composite. (a)Capacity-cycle number plots ofthe SiOx/Ccompositeand pristine Si at 0.5 A g-1. The current density for the initial two activation cycles is 0.05 A g-1.(b)Voltage profiles of the SiOx/C electrode at different cycles.(c) Rate capabilities of the SiOx/C and pristine Si electrodes at various current densities from 0.5 to 2.5 A g-1. (d) CV curves ofthe SiOx/Celectrode at different scan rates from 0.01 to 0.04 mV s-1. (e) Determination of b-value through the fitted linear model of log i and log v on redox peaks of the SiOx/C electrode.(f) Cycling performance of thegraphite-SiOx/Chybrid and pure graphite electrodes with the same areal capacity fixed at 1 mAh cm-2. The electrodes were cycled at 0.02 A g-1for the initial two activation cycles and at 0.08 A g-1for the subsequent cycles. (g)Galvanostatic voltage profiles of thegraphite-SiOx/C electrodeat different cycles.
Related work has been published on Nano Letters(Nano Lett., 2021,https://pubs.acs.org/doi/pdf/10.1021/acs.nanolett.1c00317) On March 18, 2021, with the title of "Manipulating oxidation of silicon with fresh surface enabling stable battery anode". The first unit for this research is Wuhan National Laboratory for OptoelectronicsHuazhong University of Science and Technology, and it has been funded by National Key R&D Program of China (2018YFB0905400) and Major Technological Innovation Project of Hubei Science and Technology Department (2019AAA164).