Lithium-ion batteries (LIBs) are the dominating power source for electric vehicles and have shown great promise in grid energy storage. Energy density and power density are two of their most important parameters and should be well-balanced for specific application conditions. The microstructure of an electrode, which plays a critical role in the electrochemical performance of lithium-ion batteries, including the energy and power density, could be regulated by calendaring during electrode fabrication. For electrodes comprising of a given active material, binder, and conductive additive, porosity and tortuosity are the two key parameters that dictate ion diffusion. Due to compaction density affecting electron transport in the electrode, these parameters determine the volumetric capacity and specific capacity (active materials utilization) of an electrode under specific test conditions. Therefore, effective electrode microstructure regulation will help to achieve high-performance lithium-ion batteries to meet the needs of different application scenarios.
Yongming Sun’ group utilized calendaring to fabricate electrodes of different given thicknesses that shared the same mass loading but had different electrode parameters. Using a micrometer-scale Wadsley-Roth phase TiNb2O7 active material with intercalation chemistry, they investigated the relationship between electrochemical performances and electrode microstructures by both experimental investigation and theoretical modeling, providing a paradigm of calendaring-driven electrode microstructure for balanced battery energy density and power density. Along with the reduction in porosity, ion and electron diffusion distance decreases, which is beneficial for charge transfer and rate capability. Nevertheless, the narrowed ion diffusion pathways would increase the resistance to ion diffusion. The rate capability, volumetric capacity, and materials utilization are thus predominantly restricted by the microstructures of the electrode. The dependence of electrochemical performance on porosity and electrode compaction has been investigated to provide fundamental insights into the microstructures of electrodes for different application conditions. As an example, an optimized TiNb2O7 electrode with a compaction density of ~2.5 g cm-3 and mass loading of ~8.5 mg cm-2 provided the highest specific charge capacity of 271.3 mAh g-1 with high capacity retention of 72.2% at 3C in half cell configuration and 70.4% capacity retention at 6C in full configuration. Also, a ~8 Ah level pouch cell with an optimized anode structure exhibited high capacity retention of 91.1% for 500 cycles at 0.5C.

Figure 1. (a) Lithiation potential and specific capacity for Li4Ti5O12, graphite, and TiNb2O7; (b) The comparison of the true density, specific capacity, and volumetric capacity of Li4Ti5O12, graphite, and TiNb2O7; (c) Impact of electrode thickness on the volumetric capacity of Li4Ti5O12, graphite, and TiNb2O7. (d) The volumetric energy density of LiFePO4‖TiNb2O7 and LiFePO4‖Li4Ti5O12 at 10 Ah-level.
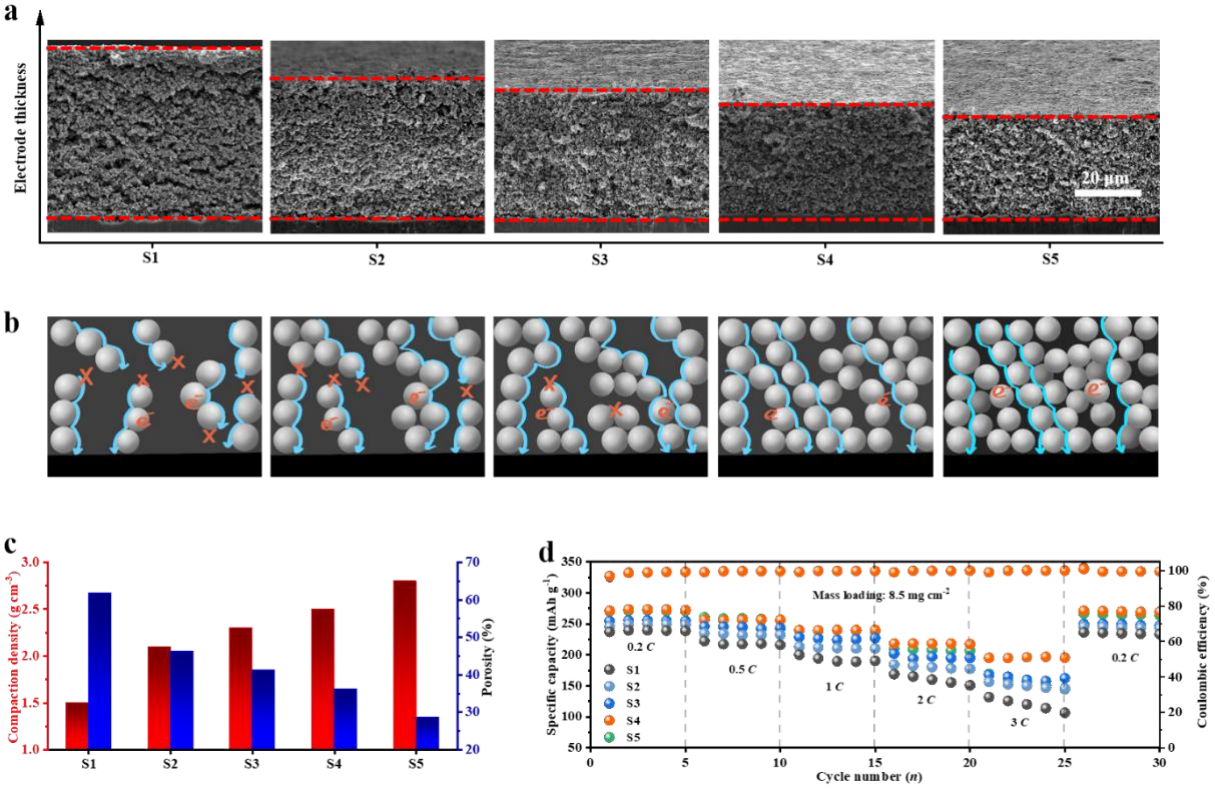
Figure 2. (a) Cross-section SEM images of TiNb2O7 electrodes with different compaction densities (S1-S5); (b) Illustration of microstructures for the S1-S5 electrodes; (c) The comparison of the compaction density, porosity and (d) rate performance for the S1-S5 electrodes.
Related work has been published on Advanced Energy Materials (https://doi.org/10.1002/aenm.202202544.) with the title A Paradigm of Calendaring-Driven Electrode Microstructure for Balanced Battery Energy Density and Power Density. The first unit for this research is the Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, and it has been funded by the National Natural Science Foundation of China.