Lithium ion batteries (LIBs) have been widely used in our life due to their high specific capacity, long cycle life and good safety performance. However, during the first charge/discharge cycle of LIBs, the anode would react with the electrolyte to form a solid electrolyte interface layer (SEI), causing the irreversible lithium loss and thus reducing the capacity and energy density of LIBs. Moreover, with the development of Si/C anode and other high-capacity anode materials for the next-generation LIBs, the low initial Coulombic efficiency of these materials will lead to more serious lithium loss, which significantly limits the energy density of LIBs. Prelithiation can compensate for this initial lithium loss in the initial cycle and improve the reversible capacity and energy density of LIBs. Prelithiation mainly includes electrochemical prelithiation, adding prelithiation additive and other methods. The prelithiation additives are divided into cathode and anode additives. Although the anode additives have high lithium ion specific capacity, their high reactivity limits the practical application. The cathode additives show better chemical stability and specific capacity for lithium compensation. Therefore, it is of vital importance to develop cathode prelithiation additives with high specific capacity and good chemical stability, which are compatible with battery manufacturing.
In view of the current research process, Professor Yongming Sun’s group (from Huazhong University of Science and Technology) took into account both the chemical stability and lithium ion specific capacity of the cathode prelithiation additives. They fabricated Fe/LiF/Li2O cathode additive with high lithium ion specific capacity (550 mAh/g) by reacting FeOF with molten lithium. It was found that Fe/LiF/Li2O could affords high lithium ion capacity through a conversion reaction (3Fe + 3LiF+ 3Li2O ® Fe2O3 + FeF3 + 9Li+ + 9e-). More importantly, the prelithiation additive only afforded lithium ions and would not be lithiated in the voltage cut-off range of LIBs (2.5-4.2 V). The Fe/LiF/Li2O additive showed good chemical stability due to the uniform distribution of LiF in the composite. The dissolution experiment suggested that no Fe dissolution was found after being soaked in commercial electrolyte for 72 hours. The Fe/LiF/Li2O additive could exhibit a specific capacity of 359mAh/g even after storing in the ambient air for 48 hours. By adding a small amount of Fe/LiF/Li2O additive into LiCoO2, LiFePO4 and LiNixCoyMn1-x-yO2 cathodes, the LIBs showed 15%-20% increase of charging capacity in comparison to the counterparts without prelithiation additive, suggesting the good lithium compensating effect of Fe/LiF/Li2O additive. Moreover, the addition of Fe/LiF/Li2O additive has no negative effect on the cycling stability. Using 4.8 wt% Fe/LiF/Li2O additive based on the total mass of the electrodes, a LiNi0.8Co0.1Mn0.1O2|SiO-graphite full cell with a high cathode mass loading of 20 mg/cm2 exhibited a high reversible capacity of 2.9 mAh/cm2 at 0.5 C after 100 cycles; a 15% increase in comparison to the counterpart without the prelithiation additive. In addition, the Fe/LiF/Li2O additive exhibited good compatibility with existing cathode materials and battery fabrication processes, which showed potential application in battery industry.
The researchers believe that this work will provide new strategies and ideas for improving the energy density of LIBs. Related work has been published on Nano Letters (Nano Letters, 2019, https://doi.org/10.1021/acs.nanolett.9b04278) On November 28, 2019, with the title of “Metal/LiF/Li2O nanocomposite for cathode prelithiation: Tradeoff between capacity and stability".
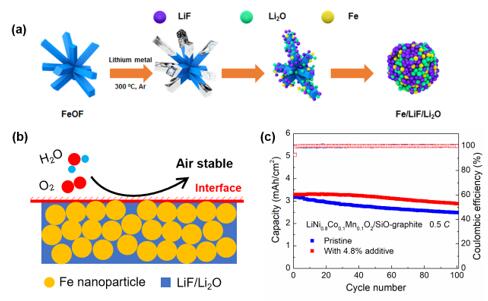
(a) Schematic of the synthesis of the Fe/LiF/Li2O nanocomposite. (b) Schematic illustration of Fe/LiF/Li2O nanocomposite exposed to the ambient air. (c) cycling stability of LiNi0.8Co0.1Mn0.1O2|SiO-graphite full cells with and without Fe/LiF/Li2O additive.