Fast-charging/discharging capability is a critical parameterfor energy storage devices applied in the field of smart gridsand sustainable energy storage, where rechargeable aqueous Zn metalbatteries are arousing increasing attention due to theadvantages of cost-effectiveness, inherent safety, and ecofriendliness.Nevertheless, Zn battery chemistry gets intolimited reversibility issues in aqueous electrolyte, as exemplifiedby the metallic Zn dendrites, low Coulombic efficiency, and then short rechargeable life, let alone the unsatisfied power density and fast-charging capability, whichare all relevant to the fundamental mechanisms on Zn electro deposition reactions. Moreover, achieving the dynamic electro deposition reaction and real-time structure information on metallic Zn under battery operating conditions can help researchers to understand the effects of various electrolytes and structures and to design advanced electrodes. Therefore, it is highly desirable to investigate the dynamic Zn electrodeposition reaction and reveal the conditions for high power application of Zn metal electrodes, which relies heavily on both in situ characterization technologies and theoretical analysis that can offer key information during the ongoing electrodeposition reaction processes.
In view of the current research process, Professor Yongming Sun’s group (from Huazhong University of Science and Technology) studied the ultrafast Zn metal electrodeposition reaction with in situ optical visualization and theoretical modeling, and uncovered that the reversibility of the Zn electrode was strongly correlated with the deposit morphology under various current densities. In a typical 1 MZnSO4 electrolyte, a non-uniform deposit featuring randomly stacked Zn nanosheets was observed at low currentdensities of 1 mA cm-2. On increasing the current density to10 mA cm-2, the deposited Zn layer became uniform with a closely packed structure. Under an ultrahigh current density of 100 mA cm-2, the concentration polarization at electrode/electrolyte interface led to the deposition of metal pillars with a tree shape. Moreover, increasing the concentration of Zn2+-ions in the electrolyte was found to be effective in widening the current density window for uniform electrochemical plating of metallic Zn. Highly stable plating/stripping behavior with a remarkable average Coulombic efficiency of >99% was achieved in a wide range of 10–100 mAcm-2 in 3 M ZnSO4 electrolyte. Theoretical calculations further indicated that the deposition modes ofmetallic Zn at various current densities depend on the competition among the crystallographic thermodynamics, kinetics, and Zn2+-ion diffusion, resulting in the deposit morphology of stacked hexagonal plates, horizontal dense granules, and vertical metal pillars, respectively. Based upon this understanding, a highly reversible Zn metal electrode with a depth of discharge of 66.7% under an ultrahigh current density of 50 mAcm-2 is achieved in both Zn||Znsymmetrical cells and MnO2||Zn full cells.
The researchers believe that the findings in this work shed light on thefundamental understanding of Zn metal nucleation andgrowth under various battery operating conditions, guidingthe design of high power Zn metal batteries with a longcycle life.Related work has been published on Angewandte Chemie International Edition (Angew. Chem. Int. Ed., 2022, DOI: 10.1002/anie.202116560) on January 27, 2022, with the title of Ultrafast Metal Electrodeposition Revealed by In Situ Optical Imaging and Theoretical Modeling towards Fast-Charging Zn Battery Chemistry.
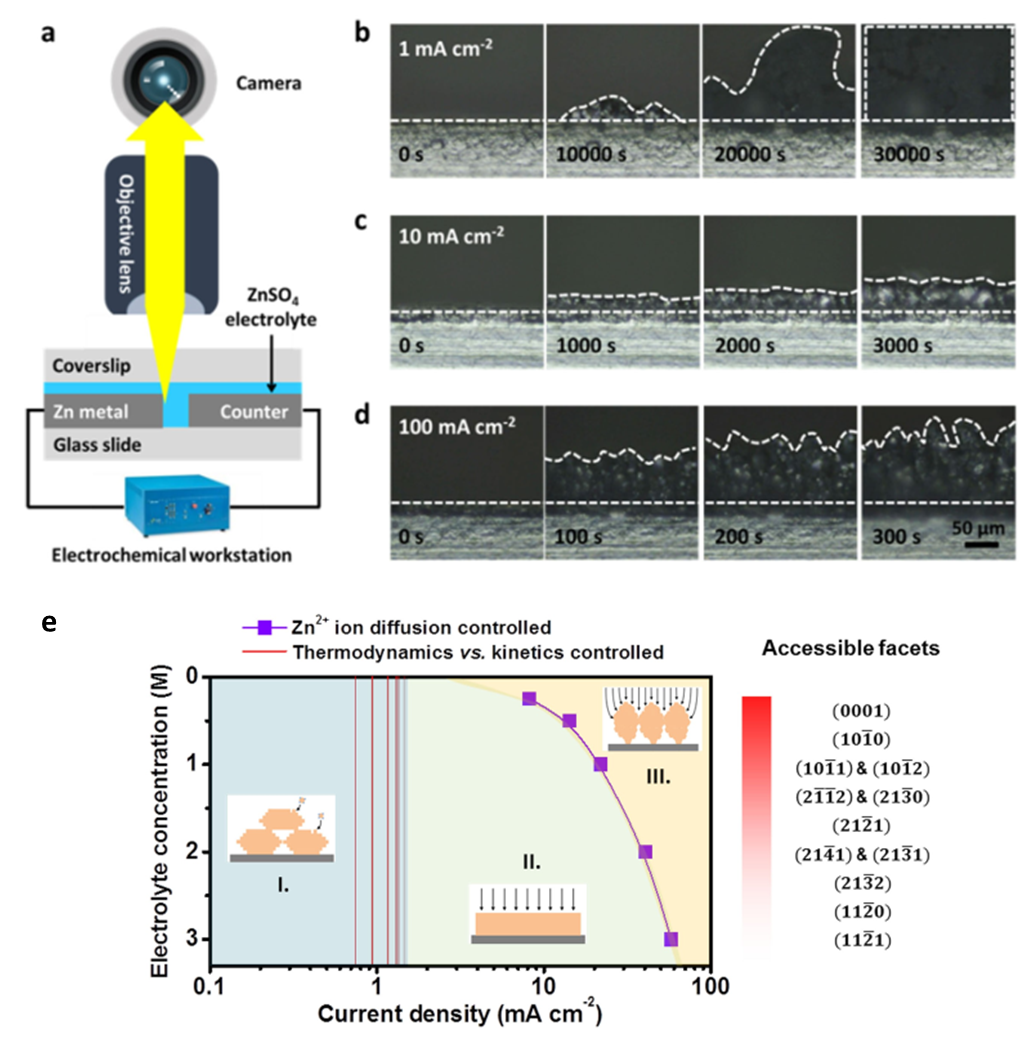
(a) Schematic of the transparent electrochemical cell for in situ optical imaging. The Zn metal electrode was covered by water-resistant epoxy to ensure the working area at cross-section. Operando OM images of plated Zn metal in ZnSO4 electrolyte at (b) 1 mA cm-2for 30000 s, (c) 10 mA cm-2for 3000 s, and (d) 100 mA cm-2for 300 s. (e) Illustration of different morphology evolution modes of Zn deposits governed by the crystallographic thermodynamics, kinetics and Zn2+-ion diffusion at different current densities and electrolyte concentrations.